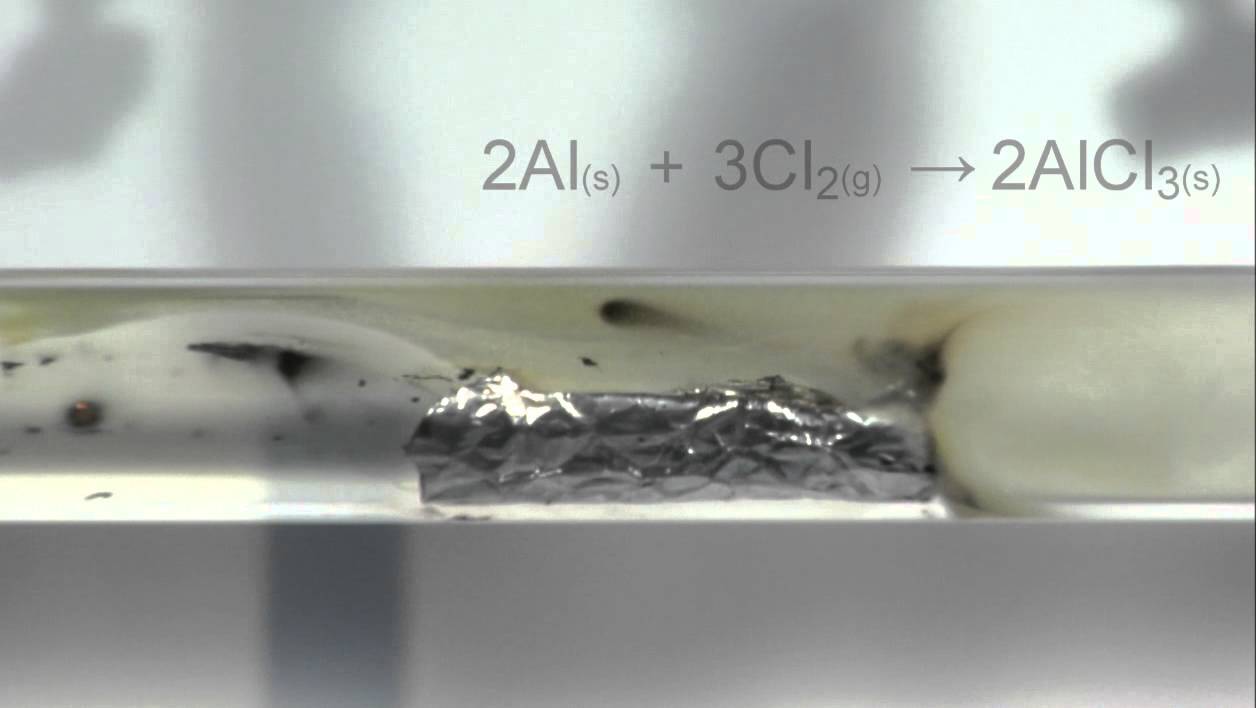
Aluminum Chloride + Oxygen . A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. To balance a chemical equation,. This oxide layer protects the metal. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Aluminum occurs almost exclusively in the +3 oxidation state. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. Aluminum oxide is insoluble in water and does. It has reactions as both a base and an acid. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For example, the reaction of aluminum with oxygen to produce aluminum oxide is.
from www.youtube.com
Aluminum occurs almost exclusively in the +3 oxidation state. This oxide layer protects the metal. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. To balance a chemical equation,. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Aluminum oxide is insoluble in water and does. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. For example, the reaction of aluminum with oxygen to produce aluminum oxide is. A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and.
Reaction of Chlorine with Aluminium YouTube
Aluminum Chloride + Oxygen Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. Aluminum oxide is insoluble in water and does. For example, the reaction of aluminum with oxygen to produce aluminum oxide is. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). This oxide layer protects the metal. Aluminum occurs almost exclusively in the +3 oxidation state. A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. To balance a chemical equation,. It has reactions as both a base and an acid.
From icsa.co.id
Poly Aluminium Chloride (PAC) Pengertian, Keuntungan, dan Aluminum Chloride + Oxygen Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. This oxide layer protects the metal. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms. Aluminum Chloride + Oxygen.
From sites.google.com
Aluminum Table of Elements by Shrenil Sharma Aluminum Chloride + Oxygen 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. To balance a chemical equation,. Aluminum occurs almost exclusively in the +3 oxidation state. Aluminum oxide is insoluble in water and does. For example, the reaction. Aluminum Chloride + Oxygen.
From jeritek.com
EDM 3 Aluminum Chloride Solution Jeritek LLC Aluminum Chloride + Oxygen Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Aluminum oxide is insoluble in water and does. It has reactions as. Aluminum Chloride + Oxygen.
From www.zxchem.com
Aluminum Chloride Hexahydrate ZXCHEM GROUP Aluminum Chloride + Oxygen Aluminum oxide is insoluble in water and does. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. Aluminum occurs almost exclusively in the +3 oxidation state. To balance. Aluminum Chloride + Oxygen.
From study.com
Aluminum Chloride AlCl3 Uses & Hazards Lesson Aluminum Chloride + Oxygen A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). It has reactions as both a base and an acid. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of. Aluminum Chloride + Oxygen.
From brainly.in
Ionic bond of aluminium chloride with diagram Brainly.in Aluminum Chloride + Oxygen It has reactions as both a base and an acid. Aluminum occurs almost exclusively in the +3 oxidation state. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively.. Aluminum Chloride + Oxygen.
From wiringdatatrujillo.z19.web.core.windows.net
Electron Dot Diagram For Aluminum Aluminum Chloride + Oxygen Aluminum oxide is insoluble in water and does. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. Aluminum occurs almost exclusively. Aluminum Chloride + Oxygen.
From www.lazada.com.my
Aluminium Chloride 6H2O AR, 500g, BENDOSEN Lazada Aluminum Chloride + Oxygen 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. For example, the reaction of aluminum with oxygen to produce aluminum oxide is. This oxide layer protects the metal. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Aluminum occurs almost exclusively in the +3 oxidation state. It. Aluminum Chloride + Oxygen.
From www.fishersci.com
Aluminum Chloride, 0.1 M Solution, Spectrum Chemical, Quantity 1 L Aluminum Chloride + Oxygen To balance a chemical equation,. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Aluminum oxide is insoluble in water and does. A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. Aluminum occurs almost exclusively in the. Aluminum Chloride + Oxygen.
From www.youtube.com
How to write Molecular formula of Aluminium chloride Chemical formula Aluminum Chloride + Oxygen This oxide layer protects the metal. Aluminum occurs almost exclusively in the +3 oxidation state. Aluminum oxide is insoluble in water and does. For example, the reaction of aluminum with oxygen to produce aluminum oxide is. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Use. Aluminum Chloride + Oxygen.
From www.youtube.com
Aluminium reacts with chlorine gas to form aluminium chloride via the Aluminum Chloride + Oxygen Aluminum oxide is insoluble in water and does. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. To balance a chemical equation,. Use the calculator below to balance. Aluminum Chloride + Oxygen.
From www.carolina.com
Aluminum Chloride, 0.2 M Solution, Aqueous, Laboratory Grade, 500 mL Aluminum Chloride + Oxygen Aluminum oxide is insoluble in water and does. A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. This oxide layer protects the metal. Therefore when aluminum foil is put into the copper salt solution, aluminum. Aluminum Chloride + Oxygen.
From www.youtube.com
Reaction of Chlorine with Aluminium YouTube Aluminum Chloride + Oxygen This oxide layer protects the metal. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. For example, the reaction of aluminum with oxygen to produce aluminum oxide is. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Aluminum. Aluminum Chloride + Oxygen.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Aluminum Chloride + Oxygen 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. It has reactions as both a base and an acid. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. Use the calculator below to balance chemical equations and. Aluminum Chloride + Oxygen.
From www.youtube.com
How to Balance AlCl3 + NaOH = Al(OH)3 + NaCl Aluminum chloride Aluminum Chloride + Oxygen Aluminum occurs almost exclusively in the +3 oxidation state. Aluminum oxide is insoluble in water and does. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. For example, the reaction of aluminum with oxygen to produce aluminum oxide is. Use the calculator below to balance chemical. Aluminum Chloride + Oxygen.
From www.slideserve.com
PPT Ionic Nomenclature PowerPoint Presentation, free download ID Aluminum Chloride + Oxygen Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. Use. Aluminum Chloride + Oxygen.
From aluminumchloridegoshiema.blogspot.com
Aluminum Chloride Electron Dot Diagram For Aluminum Chloride Aluminum Chloride + Oxygen A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. To balance a chemical equation,. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of. Aluminum Chloride + Oxygen.
From labequipsupply.co.za
Aluminum Chloride Quality Lab Chemicals RLS Chemicals Aluminum Chloride + Oxygen For example, the reaction of aluminum with oxygen to produce aluminum oxide is. It has reactions as both a base and an acid. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of. Aluminum Chloride + Oxygen.
From www.youtube.com
Reaction of Oxygen and Aluminium YouTube Aluminum Chloride + Oxygen Use the calculator below to balance chemical equations and determine the type of reaction (instructions). This oxide layer protects the metal. Aluminum oxide is insoluble in water and does. It has reactions as both a base and an acid. Aluminum occurs almost exclusively in the +3 oxidation state. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two. Aluminum Chloride + Oxygen.
From www.youtube.com
Is Al2O3 (Aluminum oxide) Ionic or Covalent? YouTube Aluminum Chloride + Oxygen 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. Aluminum oxide is insoluble in water and does. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). It has reactions as both a base and an acid. For example, the reaction of aluminum with oxygen to produce aluminum. Aluminum Chloride + Oxygen.
From aluminumchloridegoshiema.blogspot.com
Aluminum Chloride Reaction Of Aluminum Chloride With Excess Water Aluminum Chloride + Oxygen It has reactions as both a base and an acid. Aluminum occurs almost exclusively in the +3 oxidation state. To balance a chemical equation,. Aluminum oxide is insoluble in water and does. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. For example, the. Aluminum Chloride + Oxygen.
From www.adda247.com
Aluminium Chloride Formula AlCl3 Chemical Compound Name, Structure Aluminum Chloride + Oxygen 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. Aluminum oxide is insoluble in water and does. It has reactions as both a base and an acid. This oxide layer protects the metal. A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. Use the. Aluminum Chloride + Oxygen.
From ramacopetroleum.com
POLY ALUMINUM CHLORIDE (PAC) Rama Co Petroleum Aluminum Chloride + Oxygen For example, the reaction of aluminum with oxygen to produce aluminum oxide is. Aluminum occurs almost exclusively in the +3 oxidation state. A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. It has reactions as both a base and an acid. Al (clo3)3 = o2 + alcl3 is a decomposition reaction. Aluminum Chloride + Oxygen.
From byjus.com
During electrolysis of fused aluminum chloride 0.9g of Al was deposited Aluminum Chloride + Oxygen A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. This oxide layer protects the metal. It has reactions as both a base and an acid. For example, the reaction of aluminum with oxygen to produce aluminum oxide is. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons. Aluminum Chloride + Oxygen.
From www.slideserve.com
PPT Chemical Nomenclature PowerPoint Presentation, free download ID Aluminum Chloride + Oxygen It has reactions as both a base and an acid. Aluminum oxide is insoluble in water and does. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For example, the reaction of aluminum with oxygen to produce aluminum oxide is. To balance a chemical equation,. Therefore when aluminum foil is put into the copper. Aluminum Chloride + Oxygen.
From material-properties.org
Aluminium and Chlorine Comparison Properties Material Properties Aluminum Chloride + Oxygen A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. To balance a chemical equation,. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. It has reactions as both a base and an acid. This oxide layer protects. Aluminum Chloride + Oxygen.
From byjus.com
61. During electrolysis of fused aluminium chloride 0.9 g of aluminium Aluminum Chloride + Oxygen A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. This oxide layer protects the metal. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). It has reactions as both a base and an acid. Aluminum occurs almost exclusively in the +3 oxidation state. Therefore when. Aluminum Chloride + Oxygen.
From stock.adobe.com
Stylized molecule model/structural formula of aluminum chloride. Stock Aluminum Chloride + Oxygen Aluminum oxide is insoluble in water and does. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. To balance a chemical equation,. For example, the reaction of aluminum. Aluminum Chloride + Oxygen.
From www.youtube.com
Aluminum chloride reacts with barium hydroxide in an aqueous solution a Aluminum Chloride + Oxygen 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. Aluminum occurs almost exclusively in the +3 oxidation state. To balance a chemical equation,. This oxide layer protects the metal. A page showing balanced chemical equations for the reactions between sodium and oxygen, aluminium and chlorine, aluminium and. Al (clo3)3 = o2 + alcl3. Aluminum Chloride + Oxygen.
From www.adda247.com
Aluminium Chloride Formula AlCl3 Chemical Compound Name, Structure Aluminum Chloride + Oxygen Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. Aluminum occurs almost exclusively in the +3 oxidation state. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. This oxide layer protects the metal. Therefore when aluminum foil. Aluminum Chloride + Oxygen.
From www.westchem.com.au
Poly Aluminium Chloride PAC50 Industrial Coagulation Solution Aluminum Chloride + Oxygen Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For example, the reaction of aluminum with oxygen to produce aluminum oxide is. Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. This oxide layer protects the metal. It has reactions. Aluminum Chloride + Oxygen.
From www.indiamart.com
Aluminium Chloride at Rs 55/kilogram Aluminum Chloride ID 21229226612 Aluminum Chloride + Oxygen To balance a chemical equation,. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two electrons and becomes negatively. This oxide layer protects the metal. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. A page showing balanced chemical equations for the. Aluminum Chloride + Oxygen.
From www.dreamstime.com
Aluminium Chloride in Bottle , Chemical in the Laboratory and Industry Aluminum Chloride + Oxygen Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Aluminum occurs almost exclusively in the +3 oxidation state. This oxide layer protects the metal. A page showing balanced chemical equations for. Aluminum Chloride + Oxygen.
From www.flinnsci.ca
Aluminum Chloride, Reagent, 100 g Flinn Scientific Aluminum Chloride + Oxygen Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). For example, the reaction of aluminum with oxygen to produce aluminum oxide is. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each. Aluminum Chloride + Oxygen.
From www.flinnsci.com
Flinn Chemicals, Aluminum Chloride Aluminum Chloride + Oxygen Therefore when aluminum foil is put into the copper salt solution, aluminum atoms on the surface of the foil (in contact with the. Al (clo3)3 = o2 + alcl3 is a decomposition reaction where two moles of aluminum chlorate [al (clo 3) 3] decomposes into nine moles of. 4al(s) + 3o2 → 2al2o3(s) (4.4.2) each neutral oxygen atom gains two. Aluminum Chloride + Oxygen.