Experiment Preparation Of Benzoic Acid . It is a two step process: Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. The purpose of this experiment is to prepare benzoic acid through oxidation. It is also meant to demonstrate some general preparation methods,. The purpose of this experiment is to prepare and analyze benzoic acid. In the formation of benzoic acid, a grignard reagent is first prepared. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get a To prepare crystals of pure benzoic acid from an impure sample. Benzoic acid is a crystalline solid has high solubility in hot. The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. Add the impure benzoic acid to a boiling tube. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a.
from studylib.net
Benzoic acid is a crystalline solid has high solubility in hot. To prepare crystals of pure benzoic acid from an impure sample. The purpose of this experiment is to prepare and analyze benzoic acid. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get a It is also meant to demonstrate some general preparation methods,. In the formation of benzoic acid, a grignard reagent is first prepared. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. The purpose of this experiment is to prepare benzoic acid through oxidation. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method.
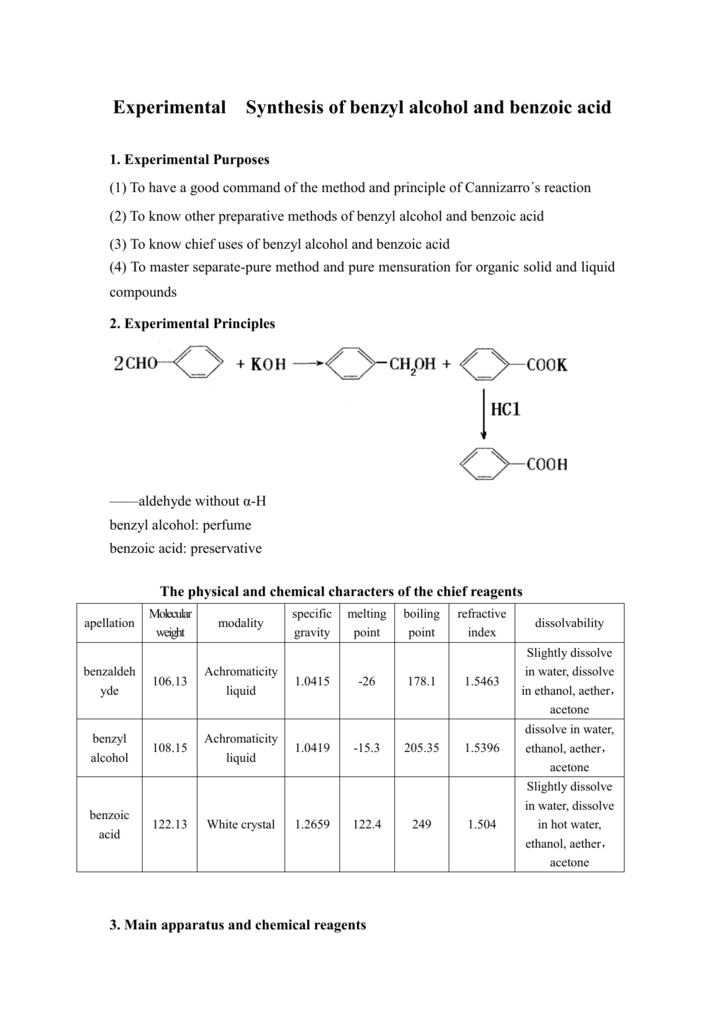
Experimental Synthesis of benzyl alcohol and benzoic acid
Experiment Preparation Of Benzoic Acid In the formation of benzoic acid, a grignard reagent is first prepared. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get a The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. To prepare crystals of pure benzoic acid from an impure sample. It is also meant to demonstrate some general preparation methods,. Add the impure benzoic acid to a boiling tube. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. In the formation of benzoic acid, a grignard reagent is first prepared. It is a two step process: The purpose of this experiment is to prepare and analyze benzoic acid. Benzoic acid is a crystalline solid has high solubility in hot. The purpose of this experiment is to prepare benzoic acid through oxidation.
From www.chegg.com
Solved EXPERIMENT 5 PREPARATION OF BENZOIC ACID Oxidation of Experiment Preparation Of Benzoic Acid It is a two step process: The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. Benzoic acid is a crystalline solid has high solubility in hot. In the first. Experiment Preparation Of Benzoic Acid.
From www.slideshare.net
Experiment 4 purification recrystallization of benzoic acid Experiment Preparation Of Benzoic Acid Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. To prepare crystals of pure benzoic acid from an impure sample. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. It is a two step process: In the formation of benzoic acid,. Experiment Preparation Of Benzoic Acid.
From www.pdfprof.com
preparation of benzoic acid by hydrolysis of ethyl benzoate Experiment Preparation Of Benzoic Acid In the formation of benzoic acid, a grignard reagent is first prepared. The purpose of this experiment is to prepare and analyze benzoic acid. It is a two step process: Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. The purpose of this experiment is to prepare benzoic acid through oxidation. To prepare. Experiment Preparation Of Benzoic Acid.
From www.studocu.com
.Experiment 5 Preparation of Benzoic Acid using a Grignard Reagent Experiment Preparation Of Benzoic Acid The purpose of this experiment is to prepare and analyze benzoic acid. The purpose of this experiment is to prepare benzoic acid through oxidation. In the formation of benzoic acid, a grignard reagent is first prepared. The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. Benzoic acid is a crystalline. Experiment Preparation Of Benzoic Acid.
From www.studocu.com
Synthesis of Benzoic Acid4 Grignard Synthesis of Benzoic Acid Experiment Preparation Of Benzoic Acid Add the impure benzoic acid to a boiling tube. To prepare crystals of pure benzoic acid from an impure sample. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get a The purpose of this experiment is to prepare benzoic acid through oxidation. In the formation of benzoic acid,. Experiment Preparation Of Benzoic Acid.
From www.studocu.com
Recrystallization of benzoic acid lab report Recrystallization of Experiment Preparation Of Benzoic Acid In the formation of benzoic acid, a grignard reagent is first prepared. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get a Benzoic acid is a crystalline solid has high solubility in hot. It is a two step process: To prepare crystals of pure benzoic acid from an. Experiment Preparation Of Benzoic Acid.
From www.numerade.com
SOLVED Experiment 12 Synthesis of Benzoic Acid prelab pts) 1) (2 pts Experiment Preparation Of Benzoic Acid Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. It is a two step process: It is also meant to demonstrate some general preparation methods,. Add the impure benzoic acid to a boiling tube. The purpose of this experiment is to prepare and analyze benzoic acid. To prepare crystals of pure benzoic acid. Experiment Preparation Of Benzoic Acid.
From cupsoguepictures.com
️ Preparation of benzoic acid. Preparation of benzoic acid from benzyl Experiment Preparation Of Benzoic Acid To prepare crystals of pure benzoic acid from an impure sample. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. The purpose of this experiment is to prepare benzoic acid through oxidation. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get. Experiment Preparation Of Benzoic Acid.
From docslib.org
Experiment 4 Preparation of Benzoic Acid DocsLib Experiment Preparation Of Benzoic Acid The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. In the formation of benzoic acid, a grignard reagent is first prepared. Benzoic acid is a crystalline solid has high solubility in hot. To prepare crystals of pure benzoic acid from an impure sample. It is a two step process: The. Experiment Preparation Of Benzoic Acid.
From studylib.net
Preparation of benzoic acid from benzamide. Experiment Preparation Of Benzoic Acid It is also meant to demonstrate some general preparation methods,. It is a two step process: In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get a Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. In. Experiment Preparation Of Benzoic Acid.
From www.studocu.com
Experiment 5 Lab Experiment 5 Experiment Title Preparation of Experiment Preparation Of Benzoic Acid It is a two step process: Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. The purpose of this experiment is to prepare and analyze benzoic acid. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. The actual laboratory we will. Experiment Preparation Of Benzoic Acid.
From www.youtube.com
Preparation of Benzoic acid from Benzamide YouTube Experiment Preparation Of Benzoic Acid The purpose of this experiment is to prepare and analyze benzoic acid. It is a two step process: In the formation of benzoic acid, a grignard reagent is first prepared. Add the impure benzoic acid to a boiling tube. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. Benzoic acid is a crystalline. Experiment Preparation Of Benzoic Acid.
From www.studocu.com
“Experiment 5 Preparation of Benzoic Acid using a Grignard Reagent Experiment Preparation Of Benzoic Acid The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. The purpose of this experiment is to prepare and analyze benzoic acid. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. Benzoic acid is a crystalline solid has high solubility in hot. It is. Experiment Preparation Of Benzoic Acid.
From studylib.net
Benzoic Acid Experiment Experiment Preparation Of Benzoic Acid The purpose of this experiment is to prepare and analyze benzoic acid. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. In the formation of benzoic acid, a grignard reagent is first prepared. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts. Experiment Preparation Of Benzoic Acid.
From www.studypool.com
SOLUTION Experiment purification and crystallization of benzoic acid Experiment Preparation Of Benzoic Acid Benzoic acid is a crystalline solid has high solubility in hot. In the formation of benzoic acid, a grignard reagent is first prepared. The purpose of this experiment is to prepare benzoic acid through oxidation. It is also meant to demonstrate some general preparation methods,. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using. Experiment Preparation Of Benzoic Acid.
From studylib.net
Experiment 9 Synthesis of Benzoic Acid Experiment Preparation Of Benzoic Acid In the formation of benzoic acid, a grignard reagent is first prepared. The purpose of this experiment is to prepare benzoic acid through oxidation. It is also meant to demonstrate some general preparation methods,. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. It is a two step. Experiment Preparation Of Benzoic Acid.
From www.youtube.com
Experiment Preparation of Benzoic acid from Ethyl benzoate. YouTube Experiment Preparation Of Benzoic Acid The purpose of this experiment is to prepare benzoic acid through oxidation. The purpose of this experiment is to prepare and analyze benzoic acid. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. To prepare crystals of pure benzoic acid from an impure sample. In the first steps,. Experiment Preparation Of Benzoic Acid.
From childhealthpolicy.vumc.org
💄 Preparation of benzoic acid from benzyl alcohol experiment. Benzoic Experiment Preparation Of Benzoic Acid Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. Add the impure benzoic acid to a boiling tube. The purpose of this experiment is to prepare benzoic acid through oxidation. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. Benzoic acid. Experiment Preparation Of Benzoic Acid.
From www.studocu.com
Experiment 5 132120 2 Experiment 5 Preparation of Benzoic Acid using Experiment Preparation Of Benzoic Acid Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get a The purpose of this experiment is to prepare benzoic acid through oxidation. Add the impure benzoic acid to a boiling tube. Benzoic. Experiment Preparation Of Benzoic Acid.
From www.thinkswap.com
Experiment 7 Prepare Benzoic Acid from Benzyl Alcohol MF010 Experiment Preparation Of Benzoic Acid To prepare crystals of pure benzoic acid from an impure sample. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. It is a two step process: Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. The purpose of this experiment is. Experiment Preparation Of Benzoic Acid.
From www.studocu.com
Experiment 6 Extraction Separation of Benzoic Acid and Phenanthrene Experiment Preparation Of Benzoic Acid The purpose of this experiment is to prepare and analyze benzoic acid. In the formation of benzoic acid, a grignard reagent is first prepared. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get a The actual laboratory we will do is the recrystallization of benzoic acid from water. Experiment Preparation Of Benzoic Acid.
From www.scribd.com
Experiment No.1 Preparation of Benzoic Acid From Toluene PDF Experiment Preparation Of Benzoic Acid Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. Add the impure benzoic acid to a boiling tube. It is also meant to demonstrate some general preparation methods,. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to. Experiment Preparation Of Benzoic Acid.
From www.academia.edu
(PDF) EXPERIMENT 2 Covalent Bonding Benzoic Acid from Ethyl Benzoate Experiment Preparation Of Benzoic Acid Benzoic acid is a crystalline solid has high solubility in hot. In the formation of benzoic acid, a grignard reagent is first prepared. It is a two step process: To prepare crystals of pure benzoic acid from an impure sample. The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. The. Experiment Preparation Of Benzoic Acid.
From www.chegg.com
Solved Experiment 12 Synthesis of Benzoic Acid Experiment Experiment Preparation Of Benzoic Acid In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to get a Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. Benzoic acid is a crystalline solid has high solubility in hot. The actual laboratory we will do. Experiment Preparation Of Benzoic Acid.
From www.youtube.com
Preparation of Benzoic acid from Benzaldehyde Msc chemistry B.K.M Experiment Preparation Of Benzoic Acid Benzoic acid is a crystalline solid has high solubility in hot. It is also meant to demonstrate some general preparation methods,. In the formation of benzoic acid, a grignard reagent is first prepared. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. Add the impure benzoic acid to. Experiment Preparation Of Benzoic Acid.
From www.slideshare.net
Experiment 4 purification recrystallization of benzoic acid Experiment Preparation Of Benzoic Acid The purpose of this experiment is to prepare benzoic acid through oxidation. To prepare crystals of pure benzoic acid from an impure sample. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. In the first steps, solid magnesium is added to form the grignard which will then react. Experiment Preparation Of Benzoic Acid.
From pharmacyinfoline.com
Benzoic acid from benzamide synthesis Pharmaceutical Chemistry Practical Experiment Preparation Of Benzoic Acid The purpose of this experiment is to prepare and analyze benzoic acid. Add the impure benzoic acid to a boiling tube. Benzoic acid is a crystalline solid has high solubility in hot. It is also meant to demonstrate some general preparation methods,. In the formation of benzoic acid, a grignard reagent is first prepared. In the first steps, solid magnesium. Experiment Preparation Of Benzoic Acid.
From www.studypool.com
SOLUTION Preparation of benzoic acid organic chemistry laboratory Experiment Preparation Of Benzoic Acid In the formation of benzoic acid, a grignard reagent is first prepared. Benzoic acid is a crystalline solid has high solubility in hot. The purpose of this experiment is to prepare benzoic acid through oxidation. Add the impure benzoic acid to a boiling tube. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a.. Experiment Preparation Of Benzoic Acid.
From www.studocu.com
Experiment 6 EXPERIMENT 6 PREPARATION OF BENZOIC ACID INTRODUCTION Experiment Preparation Of Benzoic Acid The purpose of this experiment is to prepare and analyze benzoic acid. In the formation of benzoic acid, a grignard reagent is first prepared. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. In the first steps, solid magnesium is added to form the grignard which will then react with carbon dioxide to. Experiment Preparation Of Benzoic Acid.
From www.studypool.com
SOLUTION Preparation of benzoic acid organic chemistry laboratory Experiment Preparation Of Benzoic Acid Benzoic acid is a crystalline solid has high solubility in hot. The purpose of this experiment is to prepare benzoic acid through oxidation. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. It is a two step process: The actual laboratory we will do is the recrystallization of benzoic acid from water using. Experiment Preparation Of Benzoic Acid.
From www.studypool.com
SOLUTION Benzoic acid preparation Studypool Experiment Preparation Of Benzoic Acid The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient method. Add the impure benzoic acid to a boiling tube. It is a two step process: It is also meant to demonstrate some general preparation methods,. Benzoic acid is a crystalline solid has high solubility in hot. Revision notes on 1.3.2 preparation. Experiment Preparation Of Benzoic Acid.
From studylib.net
Grignard Synthesis Preparation of Benzoic Acid Experiment Preparation Of Benzoic Acid Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. To prepare crystals of pure benzoic acid from an impure sample. The purpose of this experiment is to prepare and analyze benzoic acid. It is a two step process: In the formation of benzoic acid, a grignard reagent is first prepared. The purpose of. Experiment Preparation Of Benzoic Acid.
From studylib.net
Experimental Synthesis of benzyl alcohol and benzoic acid Experiment Preparation Of Benzoic Acid It is also meant to demonstrate some general preparation methods,. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. The purpose of this experiment is to prepare benzoic acid through oxidation. In the formation of benzoic acid, a grignard reagent is first prepared. To prepare crystals of pure benzoic acid from an impure. Experiment Preparation Of Benzoic Acid.
From www.studocu.com
Lab 5 lab manual Experiment 5 Preparation of Benzoic Acid using a Experiment Preparation Of Benzoic Acid It is also meant to demonstrate some general preparation methods,. To prepare crystals of pure benzoic acid from an impure sample. Revision notes on 1.3.2 preparation of benzoic acid for the ocr a level chemistry syllabus, written by the chemistry experts at. The actual laboratory we will do is the recrystallization of benzoic acid from water using the temperature gradient. Experiment Preparation Of Benzoic Acid.
From www.youtube.com
Assay of Benzoic Acid Experiment of Pharmaceutical Analysis Experiment Preparation Of Benzoic Acid Benzoic acid is a crystalline solid has high solubility in hot. The purpose of this experiment is to prepare benzoic acid through oxidation. Using a boiling tube in a water bath, purify the benzoic acid via recrystallisation using a. In the formation of benzoic acid, a grignard reagent is first prepared. It is a two step process: In the first. Experiment Preparation Of Benzoic Acid.