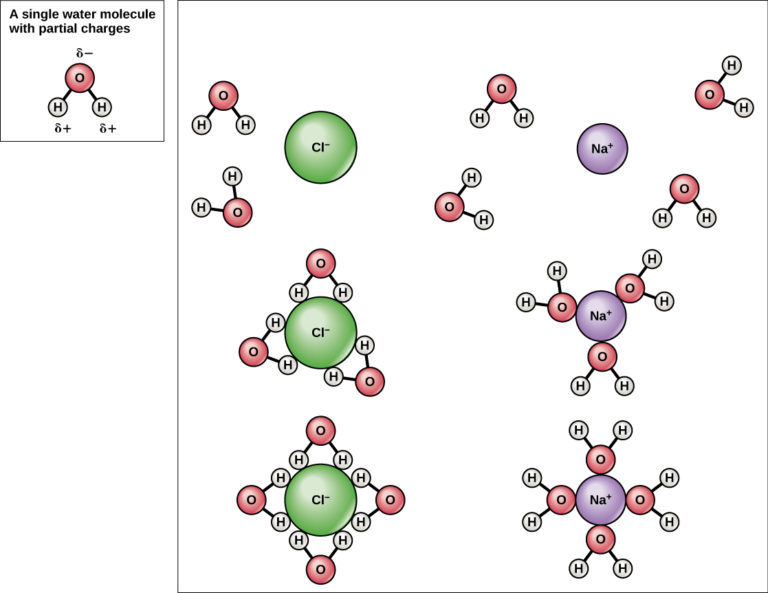
Table Salt (Nacl) And Water (H2O) Are Examples Of . Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. What do the formulas for table salt, n ac l, and water, h 2o, indicate about these compounds? \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Consider table salt (nacl, or sodium chloride): At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. Nacl is formed by transfer of one. If we take hydrochloric acid (hcl) and mix. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent.
from courses.lumenlearning.com
Nacl is formed by transfer of one. Consider table salt (nacl, or sodium chloride): At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. If we take hydrochloric acid (hcl) and mix. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. What do the formulas for table salt, n ac l, and water, h 2o, indicate about these compounds? \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +.
Water Biology I
Table Salt (Nacl) And Water (H2O) Are Examples Of If we take hydrochloric acid (hcl) and mix. At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. Nacl is formed by transfer of one. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: What do the formulas for table salt, n ac l, and water, h 2o, indicate about these compounds? If we take hydrochloric acid (hcl) and mix. Consider table salt (nacl, or sodium chloride): Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent.
From exysjisdy.blob.core.windows.net
What Is Table Salt A Compound Of at Donald McCarty blog Table Salt (Nacl) And Water (H2O) Are Examples Of Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Formation of table. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From giojwzopt.blob.core.windows.net
Science Geek Solubility Diagram at Daniel Carson blog Table Salt (Nacl) And Water (H2O) Are Examples Of Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From giohxfjle.blob.core.windows.net
When Table Salt Dissolves In Water How Does The Resulting Mixture Table Salt (Nacl) And Water (H2O) Are Examples Of At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: What do the formulas for table salt, n ac l, and water, h 2o, indicate about these compounds?. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From slideplayer.com
Chemical Reactions. ppt download Table Salt (Nacl) And Water (H2O) Are Examples Of \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: Nacl is formed by transfer of one. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Salt water, for example, is a solution of solid nacl nacl in. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From slideplayer.com
Organic Compounds Notes ppt download Table Salt (Nacl) And Water (H2O) Are Examples Of Nacl is formed by transfer of one. Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. When you dissolve table salt (sodium. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From courses.lumenlearning.com
Water Biology I Table Salt (Nacl) And Water (H2O) Are Examples Of Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Consider table salt (nacl, or sodium chloride):. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.numerade.com
a portion of the h2o nacl phase diagram is shown using the diagram Table Salt (Nacl) And Water (H2O) Are Examples Of Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. Consider table salt (nacl, or sodium chloride): At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. For example, zinc metal reacts with hydrochloric acid,. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt Table Salt (Nacl) And Water (H2O) Are Examples Of Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: Salt water, for example, is a solution of solid nacl nacl in liquid water, while. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.numerade.com
SOLVED Binary Phase Diagram SaltWater System Two Components Salt Table Salt (Nacl) And Water (H2O) Are Examples Of At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. Consider table salt (nacl, or sodium chloride): Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent. Formation of. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Table Salt (Nacl) And Water (H2O) Are Examples Of Consider table salt (nacl, or sodium chloride): Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent. If we take hydrochloric acid (hcl) and mix. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Table Salt (Nacl) And Water (H2O) Are Examples Of Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. If we take hydrochloric acid (hcl) and mix. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: When table salt is placed in water,. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From techiescientist.com
pH of NaCl — Acidic, Basic or Neutral Techiescientist Table Salt (Nacl) And Water (H2O) Are Examples Of Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. Nacl is formed by transfer of one. Formation of table salt the formation of sodium chloride (nacl) or. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From sciencenotes.org
What Is a Salt in Chemistry? Definition and Examples Table Salt (Nacl) And Water (H2O) Are Examples Of When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. If we take hydrochloric acid (hcl) and mix. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: Nacl is formed by transfer of one. When table salt is placed. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.chegg.com
Solved Table salt (NaCl) added to water dissociates into the Table Salt (Nacl) And Water (H2O) Are Examples Of When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. Consider table salt (nacl, or sodium chloride):. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From learnchemistrysaltform4.weebly.com
Qualitative analysis of salts Learn Chemistry Corner Table Salt (Nacl) And Water (H2O) Are Examples Of Nacl is formed by transfer of one. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. For example, zinc metal reacts with. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From slcc.pressbooks.pub
5.2 Water’s Interactions with Other Molecules College Biology I Table Salt (Nacl) And Water (H2O) Are Examples Of If we take hydrochloric acid (hcl) and mix. Consider table salt (nacl, or sodium chloride): For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. At the same time, the slightly electronegative chlorine portion of nacl is. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.youtube.com
Table Salt (NaCl) and Its Dissolving in Water Under a Microscope (40x Table Salt (Nacl) And Water (H2O) Are Examples Of At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. When table salt is placed in water, the slightly electropositive sodium portion is attracted. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From slideplayer.com
Water, Acids and Bases Mr. Halfen Nov ppt download Table Salt (Nacl) And Water (H2O) Are Examples Of At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.hpacmag.com
fig6bsaltmoleculesinH2O HPAC Magazine Table Salt (Nacl) And Water (H2O) Are Examples Of Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent. At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. Compounds, the are the examples of compounds as water. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From slideplayer.com
Elements, Compounds, Mixtures and Solutions ppt download Table Salt (Nacl) And Water (H2O) Are Examples Of At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. Nacl is formed by transfer of one. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From stock.adobe.com
Vecteur Stock Ionic covalent bonds examples. Chemical structural models Table Salt (Nacl) And Water (H2O) Are Examples Of \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Nacl is formed by transfer of one. If we take hydrochloric acid (hcl) and mix. Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. Salt water, for example, is a solution of solid nacl nacl in liquid water, while. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Table Salt (Nacl) And Water (H2O) Are Examples Of If we take hydrochloric acid (hcl) and mix. Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Consider table salt (nacl, or sodium chloride): Nacl is formed by transfer of one. What do. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From slideplayer.com
Solutes and Solubility ppt download Table Salt (Nacl) And Water (H2O) Are Examples Of Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. When table salt. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From slideplayer.com
Unit 10 Solutions Lecture 2 Solutions and Solubility ppt download Table Salt (Nacl) And Water (H2O) Are Examples Of When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. If we take hydrochloric acid (hcl) and mix. Formation of table salt the formation of sodium chloride (nacl). Table Salt (Nacl) And Water (H2O) Are Examples Of.
From bio1151b.nicerweb.net
solvent.html 03_07DissolvingSaltL.jpg Table Salt (Nacl) And Water (H2O) Are Examples Of If we take hydrochloric acid (hcl) and mix. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Nacl is formed by transfer of one. Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Table Salt (Nacl) And Water (H2O) Are Examples Of Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: Consider table salt (nacl, or sodium chloride): \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Nacl is formed by transfer of one. Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From joijfpoqz.blob.core.windows.net
Formation Of Table Salt Formula at John Canada blog Table Salt (Nacl) And Water (H2O) Are Examples Of For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. Consider table salt (nacl, or sodium chloride): Compounds, the are the examples of compounds as water is h2o and salt is nacl and. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Table Salt (Nacl) And Water (H2O) Are Examples Of At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. What do the formulas for table salt, n ac l, and water, h 2o, indicate about these compounds? When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. Compounds, the. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Table Salt (Nacl) And Water (H2O) Are Examples Of Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent. At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. If we take hydrochloric acid (hcl) and mix. Consider. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From philschatz.com
Compounds Essential to Human Functioning · Anatomy and Physiology Table Salt (Nacl) And Water (H2O) Are Examples Of Consider table salt (nacl, or sodium chloride): Salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o2 o 2) in a gaseous solvent. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. At the same time, the slightly electronegative chlorine portion. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Table Salt (Nacl) And Water (H2O) Are Examples Of Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. When. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.chegg.com
Solved C. Table Salt, NaCl 10. Effect of H2SO4 on table salt Table Salt (Nacl) And Water (H2O) Are Examples Of At the same time, the slightly electronegative chlorine portion of nacl is attracted to the slightly electropositive hydrogen portion of water. Nacl is formed by transfer of one. Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. If we take hydrochloric acid (hcl) and mix.. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.thoughtco.com
Chemical Composition of Table Salt Table Salt (Nacl) And Water (H2O) Are Examples Of Formation of table salt the formation of sodium chloride (nacl) or table salt is one of the most common examples of a neutralization reaction. Compounds, the are the examples of compounds as water is h2o and salt is nacl and the definition for compound is: What do the formulas for table salt, n ac l, and water, h 2o, indicate. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From rwu.pressbooks.pub
Chapter 2. The Chemical Context of Life Introduction to Molecular and Table Salt (Nacl) And Water (H2O) Are Examples Of When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen portion of water molecules. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Nacl is formed by transfer of one. Formation of table salt the formation of sodium chloride (nacl) or table salt is one of. Table Salt (Nacl) And Water (H2O) Are Examples Of.
From www.youtube.com
salt hydrolysis and net ionic equations review YouTube Table Salt (Nacl) And Water (H2O) Are Examples Of What do the formulas for table salt, n ac l, and water, h 2o, indicate about these compounds? When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change. Consider table salt (nacl, or sodium chloride): \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. If we take hydrochloric acid (hcl) and mix. When table. Table Salt (Nacl) And Water (H2O) Are Examples Of.