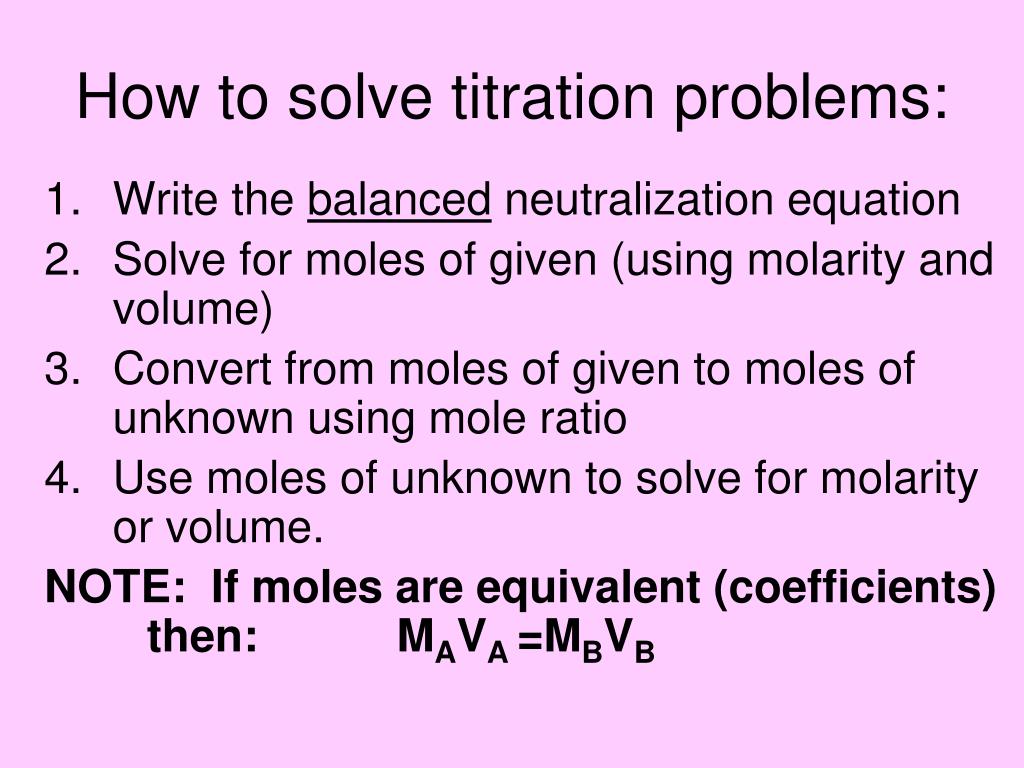
Titration Problems Explained . Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. The example below demonstrates the. The basic process involves adding a. Titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In order to titrate a substance,. When you have filled the burette and pipette, carry out the titration. Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A = nh 3, it has yet to.
from www.slideserve.com
The basic process involves adding a. The example below demonstrates the. Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. When you have filled the burette and pipette, carry out the titration. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. Titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; A = nh 3, it has yet to.
PPT Titrations PowerPoint Presentation, free download ID2145706
Titration Problems Explained In order to titrate a substance,. Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; A = nh 3, it has yet to. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. When you have filled the burette and pipette, carry out the titration. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In order to titrate a substance,. The example below demonstrates the. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know. Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. Titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). The basic process involves adding a.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Problems Explained Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In order to titrate a substance,. Before you do any titration, make sure you add an. Titration Problems Explained.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145706 Titration Problems Explained Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Titration is the procedure of identifying the quantity (moles or. Titration Problems Explained.
From dxolbdanx.blob.core.windows.net
What Is A Titration For Dummies at Jimmie Cook blog Titration Problems Explained List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. The example below demonstrates the. Place the conical flask with solution a below. Titration Problems Explained.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID3459790 Titration Problems Explained A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A = nh 3, it has yet to. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know.. Titration Problems Explained.
From www.youtube.com
Titrations Titration Calculations A level Chemistry Explained Titration Problems Explained The example below demonstrates the. A = nh 3, it has yet to. The basic process involves adding a. Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration Problems Explained.
From www.tffn.net
Solving Titration Problems A StepbyStep Guide The Enlightened Mindset Titration Problems Explained In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know. When you have filled the burette and pipette, carry out the titration. Titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the. Titration Problems Explained.
From www.youtube.com
Acidbase titration problem 1 YouTube Titration Problems Explained List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. Before you do any titration, make sure you add an indicator to the. Titration Problems Explained.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Problems Explained The example below demonstrates the. In order to titrate a substance,. When you have filled the burette and pipette, carry out the titration. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know. List the major species at. Titration Problems Explained.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Problems Explained Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. In order to titrate a substance,. The basic process involves adding a.. Titration Problems Explained.
From chem.libretexts.org
Titration of a Strong Acid With A Strong Base Chemistry LibreTexts Titration Problems Explained In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know. The basic process involves adding a. Titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a. Titration Problems Explained.
From www.youtube.com
Acid Base titration problem solving YouTube Titration Problems Explained A = nh 3, it has yet to. The example below demonstrates the. Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known. Titration Problems Explained.
From loeppodiz.blob.core.windows.net
Titration Calculation Step By Step Pdf at Linda Tibbetts blog Titration Problems Explained Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. In order to titrate a substance,. When you have filled the burette and pipette, carry out the titration. Before you do any titration, make sure you add an indicator to the solution in the conical flask. Titration Problems Explained.
From www.studocu.com
Titration Problem Solving Titration Problem Solving Example A Titration Problems Explained Titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). The example below demonstrates the. Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already. Titration Problems Explained.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration Problems Explained The example below demonstrates the. Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. The basic process involves adding a. A =. Titration Problems Explained.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Problems Explained Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. A = nh 3, it has yet to. The basic process involves adding. Titration Problems Explained.
From www.youtube.com
AP Chemistry Titration Graph problem worksheet review YouTube Titration Problems Explained The example below demonstrates the. The basic process involves adding a. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know. Before you do any titration, make sure you add an indicator to the solution in the conical. Titration Problems Explained.
From www.tffn.net
Solving Titration Problems A StepbyStep Guide The Enlightened Mindset Titration Problems Explained In order to titrate a substance,. A = nh 3, it has yet to. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. When you have filled the burette and pipette, carry out the titration. Titration is the procedure of identifying the quantity (moles or concentration) of a compound. Titration Problems Explained.
From dxohuojir.blob.core.windows.net
Titration Chemistry Problems With Solutions at Connie Stewart blog Titration Problems Explained List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. A = nh 3, it has yet to. Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; Before you do any titration,. Titration Problems Explained.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Titration Problems Explained Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; The example below demonstrates the. The basic process involves adding a. A = nh 3, it has yet to. When you have filled the burette and pipette, carry out the titration. A titration is a laboratory. Titration Problems Explained.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Problems Explained Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. The basic process involves adding a. In order to titrate a substance,. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. The. Titration Problems Explained.
From dxoaiisdq.blob.core.windows.net
Titration Explained Simply at Marlene Barron blog Titration Problems Explained List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know. In order to titrate a. Titration Problems Explained.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Problems Explained A = nh 3, it has yet to. Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. Titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a. Titration Problems Explained.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Problems Explained A = nh 3, it has yet to. The basic process involves adding a. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known. Titration Problems Explained.
From dxohuojir.blob.core.windows.net
Titration Chemistry Problems With Solutions at Connie Stewart blog Titration Problems Explained Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know. When you have filled. Titration Problems Explained.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Titration Problems Explained A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. When you have filled the burette and pipette, carry out the titration. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Titration Problems Explained.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Problems Explained Titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). In order to titrate a substance,. The basic process involves adding a. Before you do any titration, make sure you add an indicator to the solution in. Titration Problems Explained.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Problems Explained List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. The example below demonstrates the. Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. Place the conical flask with solution a below. Titration Problems Explained.
From www.slideserve.com
PPT Steps for solving titration problems PowerPoint Presentation Titration Problems Explained A = nh 3, it has yet to. The basic process involves adding a. Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; In order to titrate a substance,. The above equation works only for neutralizations in which there is a 1:1 ratio between the. Titration Problems Explained.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Problems Explained Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. When you have filled the burette and pipette, carry out the titration. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The basic process involves. Titration Problems Explained.
From dxohuojir.blob.core.windows.net
Titration Chemistry Problems With Solutions at Connie Stewart blog Titration Problems Explained Before you do any titration, make sure you add an indicator to the solution in the conical flask if you haven’t already done so. The example below demonstrates the. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already. Titration Problems Explained.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Problems Explained A = nh 3, it has yet to. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. List the major species at points a, b, c, and d on the following titration curve of the titration of ammonia with hcl. In this section,. Titration Problems Explained.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Problems Explained Titration is an analytical chemistry technique used to find an unknown concentration of an analyte (the titrand) by reacting it with a known volume and concentration of a standard solution (called the titrant). Titration is the procedure of identifying the quantity (moles or concentration) of a compound (which is called the analyte) using a well known reaction. The example below. Titration Problems Explained.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Problems Explained Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; When you have filled the burette and pipette, carry out the titration. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid. Titration Problems Explained.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration Problems Explained A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the techniques we already know. The example below demonstrates the. In order to. Titration Problems Explained.
From www.youtube.com
Titration (sample problems) YouTube Titration Problems Explained Place the conical flask with solution a below the burette, on top of a white tile which will help you visualise the end point.; When you have filled the burette and pipette, carry out the titration. A = nh 3, it has yet to. The example below demonstrates the. Before you do any titration, make sure you add an indicator. Titration Problems Explained.