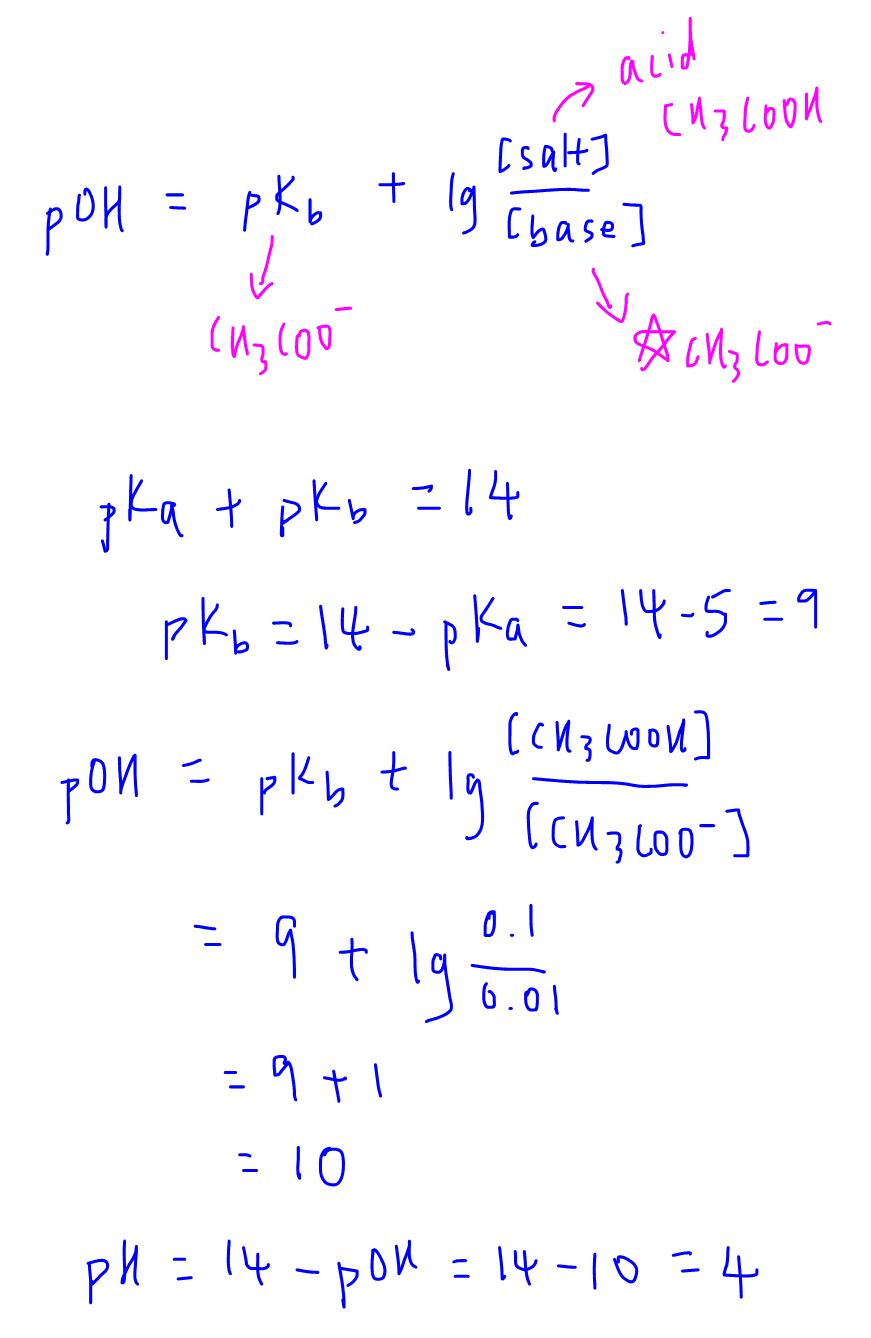Buffer Solution Calculations Examples . a buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Determine the ratio of concentrations of formate ion and formic acid in a buffer solution so that its. Β is buffer capacity (it is unitless) n is the number of. therefore, to calculate buffer capacity, we use the following formula: A buffer solution is one which. revision notes on 5.5.5 buffer calculations for the cie a level chemistry syllabus, written by the chemistry experts at save. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer. an example of an acidic buffer solution is a mixture of sodium acetate and acetic acid (ph = 4.75). For example, the following acid and base pairs work together and form buffer solutions. what is an example of a ph buffer calculation problem? examples of buffers. this page describes simple acidic and alkaline buffer solutions and explains how they work. Watch a video tutorial with examples and. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. you can calculate the ph of a buffer solution or the concentration of the acid and base using the henderson.
from chemguru.sg
Calculate the ratio of ammonium chloride to ammonia that is required to make a buffer solution with a ph of 9.00. why do these two solutions respond so differently to the addition of hcl? this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. an example of an acidic buffer solution is a mixture of sodium acetate and acetic acid (ph = 4.75). What is a buffer solution? what is an example of a ph buffer calculation problem? A salt supplies the conjugate. Suppose we needed to make a buffer solution with a ph of 2.11. buffers allow chemists to maintain a specific ph range for a reaction. Watch a video tutorial with examples and.
Calculate pH of Buffer Solution
Buffer Solution Calculations Examples this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. What is a buffer solution? A buffer solution is one which. Why is the bicarbonate buffering system important? this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer. Β is buffer capacity (it is unitless) n is the number of. Determine the ratio of concentrations of formate ion and formic acid in a buffer solution so that its. what is an example of a ph buffer calculation problem? Calculate the ratio of ammonium chloride to ammonia that is required to make a buffer solution with a ph of 9.00. For example, the following acid and base pairs work together and form buffer solutions. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. this page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. buffer solutions and ph calculations. In the first case, we would try and. you can calculate the ph of a buffer solution or the concentration of the acid and base using the henderson.
From www.youtube.com
7 Buffer Calculations 2 YouTube Buffer Solution Calculations Examples you can calculate the ph of a buffer solution or the concentration of the acid and base using the henderson. revision notes on 5.5.5 buffer calculations for the cie a level chemistry syllabus, written by the chemistry experts at save. an example of an acidic buffer solution is a mixture of sodium acetate and acetic acid (ph. Buffer Solution Calculations Examples.
From www.youtube.com
Acids and Bases Buffer Calculation Past Paper Exam Question Buffer Solution Calculations Examples buffers allow chemists to maintain a specific ph range for a reaction. therefore, to calculate buffer capacity, we use the following formula: Determine the ratio of concentrations of formate ion and formic acid in a buffer solution so that its. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. What is a buffer. Buffer Solution Calculations Examples.
From chemguru.sg
calculatephofbuffersolution Buffer Solution Calculations Examples Suppose we needed to make a buffer solution with a ph of 2.11. buffer solutions and ph calculations. buffers allow chemists to maintain a specific ph range for a reaction. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution.. Buffer Solution Calculations Examples.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Calculations Examples why do these two solutions respond so differently to the addition of hcl? In the first case, we would try and. Watch a video tutorial with examples and. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. buffer solutions and ph calculations. this is a summary practice problem set on buffer solutions. Buffer Solution Calculations Examples.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 Buffer Solution Calculations Examples buffers allow chemists to maintain a specific ph range for a reaction. Determine the ratio of concentrations of formate ion and formic acid in a buffer solution so that its. For example, the following acid and base pairs work together and form buffer solutions. this equation relates the ph, the ionization constant of a weak acid, and the. Buffer Solution Calculations Examples.
From www.youtube.com
Buffer solutions 10, lecture 2 YouTube Buffer Solution Calculations Examples Determine the ratio of concentrations of formate ion and formic acid in a buffer solution so that its. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. Suppose we needed to make a buffer solution with a ph of 2.11. In. Buffer Solution Calculations Examples.
From www.youtube.com
Buffer Preparation Calculations 01 YouTube Buffer Solution Calculations Examples Determine the ratio of concentrations of formate ion and formic acid in a buffer solution so that its. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. A salt supplies the conjugate. A mixture of acetic acid and sodium. Why is the bicarbonate buffering system important? examples of buffers. In the first case, we. Buffer Solution Calculations Examples.
From www.youtube.com
Buffer Solutions YouTube Buffer Solution Calculations Examples In the first case, we would try and. Suppose we needed to make a buffer solution with a ph of 2.11. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. A buffer is prepared containing 1.00 m acetic acid and 1.00. Buffer Solution Calculations Examples.
From www.youtube.com
How to calculate the pH of a buffer solution YouTube Buffer Solution Calculations Examples revision notes on 5.5.5 buffer calculations for the cie a level chemistry syllabus, written by the chemistry experts at save. For example, the following acid and base pairs work together and form buffer solutions. A salt supplies the conjugate. examples of buffers. this is a summary practice problem set on buffer solutions aimed to help identify buffers,. Buffer Solution Calculations Examples.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Buffer Solution Calculations Examples For example, the following acid and base pairs work together and form buffer solutions. A salt supplies the conjugate. revision notes on 5.5.5 buffer calculations for the cie a level chemistry syllabus, written by the chemistry experts at save. buffers allow chemists to maintain a specific ph range for a reaction. What is a buffer solution? this. Buffer Solution Calculations Examples.
From www.youtube.com
Buffers 3 Calculation questions on buffer solutions. YouTube Buffer Solution Calculations Examples Why is the bicarbonate buffering system important? this page describes simple acidic and alkaline buffer solutions and explains how they work. Watch a video tutorial with examples and. revision notes on 5.5.5 buffer calculations for the cie a level chemistry syllabus, written by the chemistry experts at save. A buffer is prepared containing 1.00 m acetic acid and. Buffer Solution Calculations Examples.
From www.youtube.com
Buffer Solutions PH Calculations YouTube Buffer Solution Calculations Examples buffer solutions and ph calculations. What is a buffer solution? revision notes on 5.5.5 buffer calculations for the cie a level chemistry syllabus, written by the chemistry experts at save. Why is the bicarbonate buffering system important? what is an example of a ph buffer calculation problem? this equation relates the ph, the ionization constant of. Buffer Solution Calculations Examples.
From www.youtube.com
pH of buffer solutions the HendersonHasselbalch equation. YouTube Buffer Solution Calculations Examples A buffer solution is one which. this page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. Watch a video tutorial with examples and. A mixture of acetic acid and sodium. what is an example of a ph buffer calculation problem?. Buffer Solution Calculations Examples.
From dandkmotorsports.com
Tris Buffer Recipe Calculator Dandk Organizer Buffer Solution Calculations Examples A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. why do these two solutions respond so differently to the addition of hcl? Suppose we needed to make a buffer solution with a ph of 2.11. A buffer solution is one which. A salt supplies the conjugate. revision notes on 5.5.5 buffer calculations for. Buffer Solution Calculations Examples.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer Solution Calculations Examples A mixture of acetic acid and sodium. an example of an acidic buffer solution is a mixture of sodium acetate and acetic acid (ph = 4.75). For example, the following acid and base pairs work together and form buffer solutions. this page describes simple acidic and alkaline buffer solutions and explains how they work. buffer solutions and. Buffer Solution Calculations Examples.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer Solution Calculations Examples Why is the bicarbonate buffering system important? In the first case, we would try and. buffer solutions and ph calculations. Β is buffer capacity (it is unitless) n is the number of. Determine the ratio of concentrations of formate ion and formic acid in a buffer solution so that its. examples of buffers. Suppose we needed to make. Buffer Solution Calculations Examples.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download Buffer Solution Calculations Examples buffer solutions and ph calculations. A salt supplies the conjugate. therefore, to calculate buffer capacity, we use the following formula: Β is buffer capacity (it is unitless) n is the number of. Watch a video tutorial with examples and. Suppose we needed to make a buffer solution with a ph of 2.11. this page describes simple acidic. Buffer Solution Calculations Examples.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Buffer Solution Calculations Examples Why is the bicarbonate buffering system important? examples of buffers. Determine the ratio of concentrations of formate ion and formic acid in a buffer solution so that its. What is a buffer solution? you can calculate the ph of a buffer solution or the concentration of the acid and base using the henderson. For example, the following acid. Buffer Solution Calculations Examples.
From www.youtube.com
How to Calculate PH of Buffer Solution YouTube Buffer Solution Calculations Examples Why is the bicarbonate buffering system important? buffer solutions and ph calculations. Watch a video tutorial with examples and. buffers allow chemists to maintain a specific ph range for a reaction. Suppose we needed to make a buffer solution with a ph of 2.11. what is an example of a ph buffer calculation problem? an example. Buffer Solution Calculations Examples.
From dxogneoug.blob.core.windows.net
Dilution Of Buffer Solution Effect On Ph at Tanya Elliot blog Buffer Solution Calculations Examples this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. A salt supplies the conjugate. buffer solutions and ph calculations. an example of an acidic buffer solution is a mixture of sodium acetate and acetic acid (ph = 4.75). For. Buffer Solution Calculations Examples.
From www.aakash.ac.in
Acidic Buffer Solution Definition, Calculation & Applications Buffer Solution Calculations Examples therefore, to calculate buffer capacity, we use the following formula: buffers allow chemists to maintain a specific ph range for a reaction. what is an example of a ph buffer calculation problem? buffer solutions and ph calculations. Calculate the ratio of ammonium chloride to ammonia that is required to make a buffer solution with a ph. Buffer Solution Calculations Examples.
From www.youtube.com
17.2 Calculating pH of Buffer Solutions YouTube Buffer Solution Calculations Examples this page describes simple acidic and alkaline buffer solutions and explains how they work. therefore, to calculate buffer capacity, we use the following formula: For example, the following acid and base pairs work together and form buffer solutions. you can calculate the ph of a buffer solution or the concentration of the acid and base using the. Buffer Solution Calculations Examples.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID Buffer Solution Calculations Examples What is a buffer solution? Why is the bicarbonate buffering system important? Watch a video tutorial with examples and. For example, the following acid and base pairs work together and form buffer solutions. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered. Buffer Solution Calculations Examples.
From chemguru.sg
Calculate pH of Buffer Solution Buffer Solution Calculations Examples Suppose we needed to make a buffer solution with a ph of 2.11. Β is buffer capacity (it is unitless) n is the number of. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. A mixture of acetic acid and sodium.. Buffer Solution Calculations Examples.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Solution Calculations Examples For example, the following acid and base pairs work together and form buffer solutions. Suppose we needed to make a buffer solution with a ph of 2.11. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. an example of an. Buffer Solution Calculations Examples.
From www.studypool.com
SOLUTION Buffer solution calculations Studypool Buffer Solution Calculations Examples this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. Calculate the ratio of ammonium chloride to ammonia that is required to make a buffer solution with a ph of 9.00. revision notes on 5.5.5 buffer calculations for the cie a. Buffer Solution Calculations Examples.
From www.youtube.com
pH of Buffer Solution (Example) YouTube Buffer Solution Calculations Examples a buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. therefore, to calculate buffer capacity, we use. Buffer Solution Calculations Examples.
From www.youtube.com
Buffer Calculations YouTube Buffer Solution Calculations Examples therefore, to calculate buffer capacity, we use the following formula: revision notes on 5.5.5 buffer calculations for the cie a level chemistry syllabus, written by the chemistry experts at save. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer. A salt supplies the conjugate. . Buffer Solution Calculations Examples.
From www.youtube.com
8 Buffer Calculations 3 YouTube Buffer Solution Calculations Examples this page describes simple acidic and alkaline buffer solutions and explains how they work. Β is buffer capacity (it is unitless) n is the number of. For example, the following acid and base pairs work together and form buffer solutions. In the first case, we would try and. buffer solutions and ph calculations. A buffer solution is one. Buffer Solution Calculations Examples.
From criticalthinking.cloud
buffer solution questions a level chemistry Buffer Solution Calculations Examples A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. In the first case, we would try and. A mixture of acetic acid and sodium. a buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Β is buffer capacity (it is unitless). Buffer Solution Calculations Examples.
From www.slideshare.net
2012 topic 18 2 buffer solutions Buffer Solution Calculations Examples Β is buffer capacity (it is unitless) n is the number of. buffer solutions and ph calculations. buffers allow chemists to maintain a specific ph range for a reaction. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer. examples of buffers. Watch a video. Buffer Solution Calculations Examples.
From www.studypool.com
SOLUTION Buffer solution calculations Studypool Buffer Solution Calculations Examples examples of buffers. A buffer is prepared containing 1.00 m acetic acid and 1.00 m sodium acetate. this equation relates the ph, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. buffer solutions and ph calculations. a buffer solution is one which resists. Buffer Solution Calculations Examples.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Buffer Solution Calculations Examples what is an example of a ph buffer calculation problem? why do these two solutions respond so differently to the addition of hcl? an example of an acidic buffer solution is a mixture of sodium acetate and acetic acid (ph = 4.75). therefore, to calculate buffer capacity, we use the following formula: you can calculate. Buffer Solution Calculations Examples.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps Buffer Solution Calculations Examples Calculate the ratio of ammonium chloride to ammonia that is required to make a buffer solution with a ph of 9.00. Suppose we needed to make a buffer solution with a ph of 2.11. this is a summary practice problem set on buffer solutions aimed to help identify buffers, calculating the ph of a buffer. Why is the bicarbonate. Buffer Solution Calculations Examples.
From www.youtube.com
CALCULATION pH OF BUFFER SOLUTION EXAMPLE 2 YouTube Buffer Solution Calculations Examples Β is buffer capacity (it is unitless) n is the number of. A buffer solution is one which. Calculate the ratio of ammonium chloride to ammonia that is required to make a buffer solution with a ph of 9.00. In the first case, we would try and. an example of an acidic buffer solution is a mixture of sodium. Buffer Solution Calculations Examples.