What Is Acid Base Titration . Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. The concentration of a solution can be determined by knowing the acid and base dissociation constant. there are two basic types of acid base titrations, indicator and potentiometric. Acid is titrated with a base and base is titrated with an acid. When a redox reaction is used,. The analyte (titrand) is the solution with an unknown molarity. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. In its simplest form, titration is carried out by measuring the volume of the solution. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. Compute sample ph at important stages of a titration. an acid base titration is a quantitative analysis method for estimating the concentration of an acid or base by. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong. Titration is a common laboratory technique for determining the concentration of a solute.
from mungfali.com
in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Compute sample ph at important stages of a titration. an acid base titration is a quantitative analysis method for estimating the concentration of an acid or base by. The analyte (titrand) is the solution with an unknown molarity. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. Acid is titrated with a base and base is titrated with an acid. In its simplest form, titration is carried out by measuring the volume of the solution. Titration is a common laboratory technique for determining the concentration of a solute. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak.
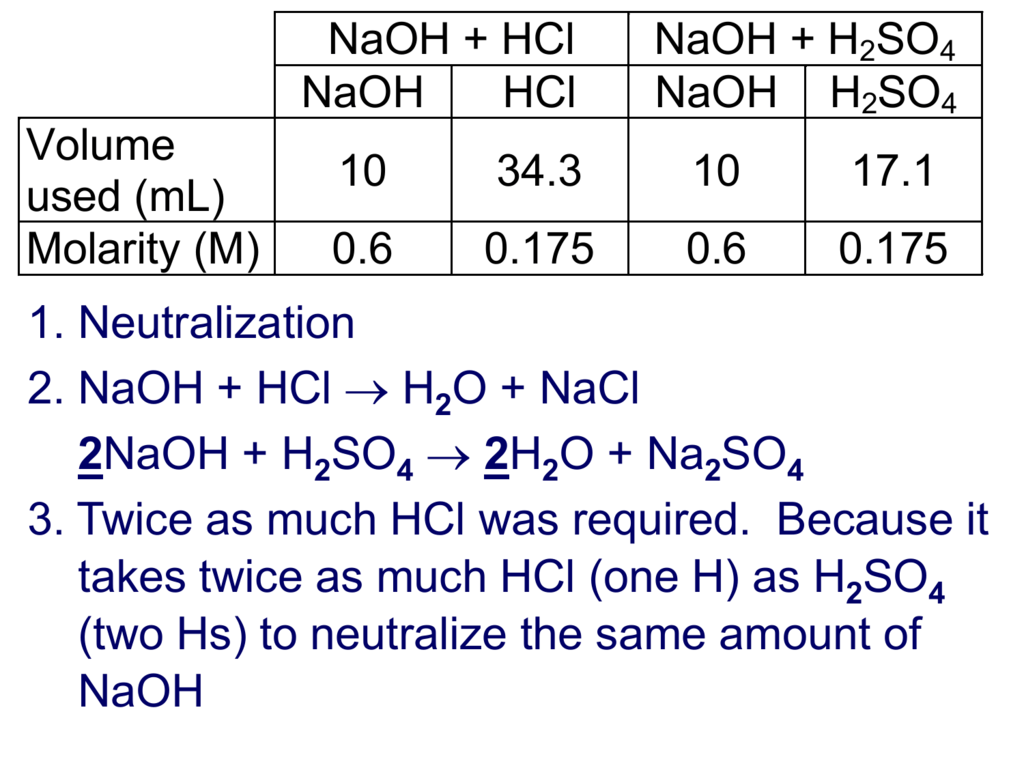
Acid Base Titration Calculation
What Is Acid Base Titration Titration is a common laboratory technique for determining the concentration of a solute. The concentration of a solution can be determined by knowing the acid and base dissociation constant. there are two basic types of acid base titrations, indicator and potentiometric. When a redox reaction is used,. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. The analyte (titrand) is the solution with an unknown molarity. an acid base titration is a quantitative analysis method for estimating the concentration of an acid or base by. In its simplest form, titration is carried out by measuring the volume of the solution. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. Acid is titrated with a base and base is titrated with an acid. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. Compute sample ph at important stages of a titration. Titration is a common laboratory technique for determining the concentration of a solute. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong. It is based on the neutralization reaction, where an acid and a base react to form water and a salt.
From schoolbag.info
Figure 10.11. Strong Acid and Weak Base Titration Curve A strong acid What Is Acid Base Titration Compute sample ph at important stages of a titration. there are two basic types of acid base titrations, indicator and potentiometric. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. Acid is titrated with a base and base is titrated with an acid. by determining the volume. What Is Acid Base Titration.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts What Is Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. In its simplest form, titration is carried out by measuring the volume of the solution. Compute sample ph at important stages. What Is Acid Base Titration.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph What Is Acid Base Titration In its simplest form, titration is carried out by measuring the volume of the solution. When a redox reaction is used,. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. in a titration, a solution of known concentration (the titrant) is added to a solution. What Is Acid Base Titration.
From mungfali.com
Acid Base Titration Lab What Is Acid Base Titration in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. The concentration of a solution can be determined by knowing the acid and base dissociation constant. by determining. What Is Acid Base Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators What Is Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. When a redox reaction is used,. there are two basic types of acid base titrations, indicator and potentiometric. In its simplest form, titration is carried out. What Is Acid Base Titration.
From www.biorender.com
AcidBase Titration BioRender Science Templates What Is Acid Base Titration there are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Compute sample ph at important stages of a titration. When a redox reaction is used,. The concentration. What Is Acid Base Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps What Is Acid Base Titration titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. Compute sample ph at important stages of a titration. Acid is titrated with a base. What Is Acid Base Titration.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector What Is Acid Base Titration by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic. What Is Acid Base Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during What Is Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Compute sample ph at important stages of a titration. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. an acid base titration is a quantitative. What Is Acid Base Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators What Is Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. there are two basic types of acid base titrations, indicator and potentiometric. The concentration of a solution can be determined by knowing the acid and base dissociation constant. Hundreds of compounds both organic and inorganic can be determined by. What Is Acid Base Titration.
From saylordotorg.github.io
AcidBase Titrations What Is Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration is a common laboratory technique for determining the concentration of a solute. Acid is titrated with a base and base is titrated with an acid. an acid base. What Is Acid Base Titration.
From mungfali.com
Acid Base Titration Calculation What Is Acid Base Titration in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. Titration is a common laboratory technique for determining the concentration of a solute. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. by determining the volume of. What Is Acid Base Titration.
From www.sliderbase.com
Titration What Is Acid Base Titration In its simplest form, titration is carried out by measuring the volume of the solution. an acid base titration is a quantitative analysis method for estimating the concentration of an acid or base by. Titration is a common laboratory technique for determining the concentration of a solute. titration of a strong acid with a strong base is the. What Is Acid Base Titration.
From webmis.highland.cc.il.us
AcidBase Titrations What Is Acid Base Titration Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. The analyte (titrand) is the solution with an unknown molarity. Compute sample ph at important stages of a titration. The concentration of a solution can be determined by knowing the acid and base dissociation constant. When a redox reaction is. What Is Acid Base Titration.
From uwaterloo.ca
Classic acid and base titration in pink Chem 13 News Magazine What Is Acid Base Titration by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. When a redox reaction is used,. Acid is titrated with a base and base is titrated with an acid. Compute sample ph at important stages of a titration. The analyte (titrand) is the solution with an unknown. What Is Acid Base Titration.
From facts.net
8 Captivating Facts About AcidBase Titration What Is Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. an acid base titration is a quantitative analysis method for estimating the concentration of an acid or base by. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. in a titration, a solution of known concentration (the. What Is Acid Base Titration.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom What Is Acid Base Titration in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. Titration is a common laboratory technique for determining the concentration of a solute. an acid base titration is a quantitative analysis method for estimating the concentration of an acid or base by. Compute sample ph at important stages. What Is Acid Base Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during What Is Acid Base Titration Acid is titrated with a base and base is titrated with an acid. When a redox reaction is used,. Compute sample ph at important stages of a titration. there are two basic types of acid base titrations, indicator and potentiometric. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance. What Is Acid Base Titration.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps What Is Acid Base Titration in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. When a redox reaction is used,. an acid base titration is a quantitative analysis method for estimating the. What Is Acid Base Titration.
From mungfali.com
Acid Base Titration Calculation What Is Acid Base Titration in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. The concentration of a solution can be determined by knowing the acid and base dissociation constant.. What Is Acid Base Titration.
From mungfali.com
Acid Base Titration Graph What Is Acid Base Titration Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being. What Is Acid Base Titration.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit What Is Acid Base Titration In its simplest form, titration is carried out by measuring the volume of the solution. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong. The analyte (titrand) is the solution with an unknown molarity. Hundreds of compounds both organic and inorganic can be determined by. What Is Acid Base Titration.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps What Is Acid Base Titration in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. The analyte (titrand) is the solution with an unknown molarity. Compute sample ph at important stages of a titration. When a redox reaction is used,. there are two basic types of acid base titrations, indicator and potentiometric. . What Is Acid Base Titration.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and What Is Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. In its simplest. What Is Acid Base Titration.
From mungfali.com
Acid Base Titration Procedure What Is Acid Base Titration The analyte (titrand) is the solution with an unknown molarity. Compute sample ph at important stages of a titration. there are two basic types of acid base titrations, indicator and potentiometric. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. It is based on the neutralization reaction,. What Is Acid Base Titration.
From mungfali.com
Acid Base Titration Calculation What Is Acid Base Titration an acid base titration is a quantitative analysis method for estimating the concentration of an acid or base by. When a redox reaction is used,. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. In its simplest form, titration is carried out by measuring the volume of the. What Is Acid Base Titration.
From www.youtube.com
Introduction to Acid Base Titrations YouTube What Is Acid Base Titration there are two basic types of acid base titrations, indicator and potentiometric. Compute sample ph at important stages of a titration. Titration is a common laboratory technique for determining the concentration of a solute. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied. It is based on. What Is Acid Base Titration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube What Is Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The analyte (titrand) is the solution with an unknown molarity. Acid is titrated with a base and base is titrated with an acid. by determining the volume of strong based needed to reach the equivalence point, the molecular weight. What Is Acid Base Titration.
From www.sciencephoto.com
AcidBase Titration Stock Image C002/8122 Science Photo Library What Is Acid Base Titration Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong. It is based on the neutralization reaction, where an acid and a base react to form. What Is Acid Base Titration.
From ar.inspiredpencil.com
Acid Base Titration What Is Acid Base Titration It is based on the neutralization reaction, where an acid and a base react to form water and a salt. there are two basic types of acid base titrations, indicator and potentiometric. titration of a strong acid with a strong base is the simplest of the four types of titrations as it involves a strong. an acid. What Is Acid Base Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators What Is Acid Base Titration In its simplest form, titration is carried out by measuring the volume of the solution. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. Acid is titrated with a base and base is titrated with an acid. by determining the volume of strong based needed to reach the. What Is Acid Base Titration.
From saylordotorg.github.io
AcidBase Titrations What Is Acid Base Titration Compute sample ph at important stages of a titration. The analyte (titrand) is the solution with an unknown molarity. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. Titration is a common laboratory technique for determining the concentration of a solute. The concentration of a solution can be determined. What Is Acid Base Titration.
From saylordotorg.github.io
AcidBase Titrations What Is Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. Titration is a common laboratory technique for determining the concentration of a solute. in a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied.. What Is Acid Base Titration.
From mungfali.com
Acid Base Titration Calculation What Is Acid Base Titration The concentration of a solution can be determined by knowing the acid and base dissociation constant. Acid is titrated with a base and base is titrated with an acid. there are two basic types of acid base titrations, indicator and potentiometric. In its simplest form, titration is carried out by measuring the volume of the solution. Hundreds of compounds. What Is Acid Base Titration.
From mungfali.com
Acid Base Titration Experiment What Is Acid Base Titration by determining the volume of strong based needed to reach the equivalence point, the molecular weight and/or pk a of the weak. Hundreds of compounds both organic and inorganic can be determined by a titration based on their acidic or basic properties. in a titration, a solution of known concentration (the titrant) is added to a solution of. What Is Acid Base Titration.