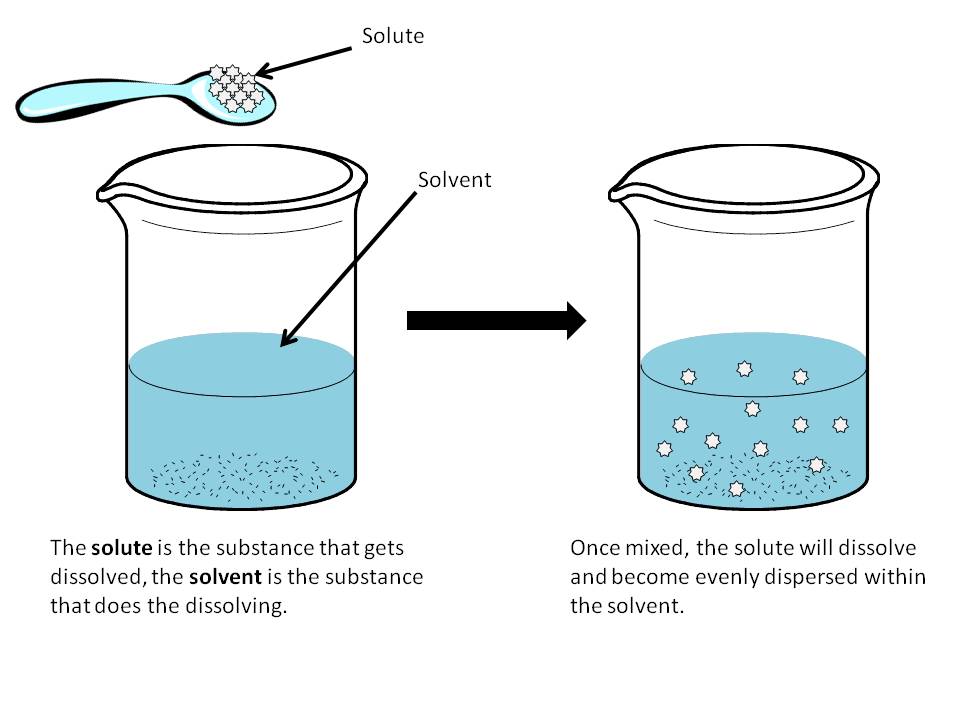
Does Vinegar Dissolve In Water True Or False . Vinegar is a dilute solution of acetic acid, which is a weak acid. In some salad dressings a layer of oil, like canola. as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving the appearance of a soluble. In fact, vinegar is very soluble in. water is not very attracted to the oil and so does not dissolve it. technically, it doesn't dissolve; Instead, it mixes with water to create a homogenous solution. Due to its strong polarity, water. • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. Can it dissolve in water? No, vinegar is not insoluble in water. the short answer is yes, vinegar does dissolve in water. In the question, the offered substance is vinegar, which is hydrophilic. is vinegar completely insoluble in water? vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic).
from sciencemsqblog8.blogspot.com
is vinegar completely insoluble in water? Can it dissolve in water? • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. Instead, it mixes with water to create a homogenous solution. as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving the appearance of a soluble. In some salad dressings a layer of oil, like canola. Vinegar is a dilute solution of acetic acid, which is a weak acid. Due to its strong polarity, water. No, vinegar is not insoluble in water. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic).
Science8 Semester 2,Chapter 4 Mixtures
Does Vinegar Dissolve In Water True Or False Due to its strong polarity, water. technically, it doesn't dissolve; In the question, the offered substance is vinegar, which is hydrophilic. No, vinegar is not insoluble in water. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). the short answer is yes, vinegar does dissolve in water. Vinegar is a dilute solution of acetic acid, which is a weak acid. is vinegar completely insoluble in water? In fact, vinegar is very soluble in. Due to its strong polarity, water. water is not very attracted to the oil and so does not dissolve it. as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving the appearance of a soluble. Can it dissolve in water? In some salad dressings a layer of oil, like canola. • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. Instead, it mixes with water to create a homogenous solution.
From www.youtube.com
Does vinegar dissolve stickers? YouTube Does Vinegar Dissolve In Water True Or False In fact, vinegar is very soluble in. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving the appearance of a soluble. Vinegar is a dilute solution of acetic acid, which is a weak acid. In some. Does Vinegar Dissolve In Water True Or False.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Does Vinegar Dissolve In Water True Or False In fact, vinegar is very soluble in. Vinegar is a dilute solution of acetic acid, which is a weak acid. is vinegar completely insoluble in water? vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). technically, it doesn't dissolve; Due to its strong polarity, water. In some salad dressings a layer. Does Vinegar Dissolve In Water True Or False.
From paityn-has-lester.blogspot.com
Which Best Dissolve Best in Water Salt or Baking Soda PaitynhasLester Does Vinegar Dissolve In Water True Or False the short answer is yes, vinegar does dissolve in water. In fact, vinegar is very soluble in. Instead, it mixes with water to create a homogenous solution. No, vinegar is not insoluble in water. water is not very attracted to the oil and so does not dissolve it. as a result, it doesn't dissolve in water but. Does Vinegar Dissolve In Water True Or False.
From sugarandcinnamon.com
Does Baking Soda Dissolve In Water? (What You Should Know Does Vinegar Dissolve In Water True Or False Instead, it mixes with water to create a homogenous solution. the short answer is yes, vinegar does dissolve in water. as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving the appearance of a soluble. Vinegar is a dilute solution of acetic acid, which is a weak acid. vinegar is a. Does Vinegar Dissolve In Water True Or False.
From www.thoughtco.com
Dissolve Definition in Chemistry Does Vinegar Dissolve In Water True Or False In some salad dressings a layer of oil, like canola. as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving the appearance of a soluble. Instead, it mixes with water to create a homogenous solution. water is not very attracted to the oil and so does not dissolve it. Due to its. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Experiment Does Chalk dissolve in Water,Vinegar And Lemon or not Does Vinegar Dissolve In Water True Or False water is not very attracted to the oil and so does not dissolve it. Instead, it mixes with water to create a homogenous solution. In the question, the offered substance is vinegar, which is hydrophilic. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). the short answer is yes, vinegar does. Does Vinegar Dissolve In Water True Or False.
From www.mytidycorner.com
Vinegar to Water Ratio for Cleaning Different Surfaces and Things Does Vinegar Dissolve In Water True Or False water is not very attracted to the oil and so does not dissolve it. Vinegar is a dilute solution of acetic acid, which is a weak acid. the short answer is yes, vinegar does dissolve in water. Instead, it mixes with water to create a homogenous solution. as a result, it doesn't dissolve in water but absorbs. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Why Does This Powder Only Dissolve In Cold Water? YouTube Does Vinegar Dissolve In Water True Or False technically, it doesn't dissolve; as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving the appearance of a soluble. Instead, it mixes with water to create a homogenous solution. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). water is not very attracted to. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Does Vinegar Dissolve Fiberglass? YouTube Does Vinegar Dissolve In Water True Or False is vinegar completely insoluble in water? as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving the appearance of a soluble. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). In fact, vinegar is very soluble in. the short answer is yes, vinegar does. Does Vinegar Dissolve In Water True Or False.
From awesomefishcollections.blogspot.com
6+ Info For Does Vinegar Dissolve Sea Urchin Spines [Updated] Awesome Does Vinegar Dissolve In Water True Or False Instead, it mixes with water to create a homogenous solution. In fact, vinegar is very soluble in. • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. Due to its strong polarity, water. Vinegar is a dilute solution of acetic acid, which is a weak acid. is vinegar completely insoluble in water? water. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Chemical reaction of chalk in water YouTube Does Vinegar Dissolve In Water True Or False vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). Instead, it mixes with water to create a homogenous solution. No, vinegar is not insoluble in water. In the question, the offered substance is vinegar, which is hydrophilic. as a result, it doesn't dissolve in water but absorbs water on a molecular level,. Does Vinegar Dissolve In Water True Or False.
From foodstruct.com
Vinegar vs. Water — InDepth Nutrition Comparison Does Vinegar Dissolve In Water True Or False water is not very attracted to the oil and so does not dissolve it. Vinegar is a dilute solution of acetic acid, which is a weak acid. the short answer is yes, vinegar does dissolve in water. No, vinegar is not insoluble in water. Due to its strong polarity, water. vinegar is a polar substance, and its. Does Vinegar Dissolve In Water True Or False.
From dxojhbucn.blob.core.windows.net
Bleach And Vinegar Reaction Equation at Herman Mathews blog Does Vinegar Dissolve In Water True Or False the short answer is yes, vinegar does dissolve in water. In fact, vinegar is very soluble in. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). is vinegar completely insoluble in water? • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. Can it dissolve in. Does Vinegar Dissolve In Water True Or False.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize Does Vinegar Dissolve In Water True Or False In the question, the offered substance is vinegar, which is hydrophilic. Instead, it mixes with water to create a homogenous solution. water is not very attracted to the oil and so does not dissolve it. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). Due to its strong polarity, water. technically,. Does Vinegar Dissolve In Water True Or False.
From yesdirt.com
Does Salt Dissolve In Vinegar? (ANSWERED) Yes Dirt Does Vinegar Dissolve In Water True Or False the short answer is yes, vinegar does dissolve in water. water is not very attracted to the oil and so does not dissolve it. In fact, vinegar is very soluble in. Due to its strong polarity, water. In the question, the offered substance is vinegar, which is hydrophilic. technically, it doesn't dissolve; Instead, it mixes with water. Does Vinegar Dissolve In Water True Or False.
From foodly.tn
Does vinegar dissolve grease? Does Vinegar Dissolve In Water True Or False is vinegar completely insoluble in water? the short answer is yes, vinegar does dissolve in water. water is not very attracted to the oil and so does not dissolve it. technically, it doesn't dissolve; In some salad dressings a layer of oil, like canola. Can it dissolve in water? Due to its strong polarity, water. Vinegar. Does Vinegar Dissolve In Water True Or False.
From www.leaf.tv
Does Vinegar Dissolve in Water? LEAFtv Does Vinegar Dissolve In Water True Or False Instead, it mixes with water to create a homogenous solution. is vinegar completely insoluble in water? Can it dissolve in water? In the question, the offered substance is vinegar, which is hydrophilic. technically, it doesn't dissolve; No, vinegar is not insoluble in water. Vinegar is a dilute solution of acetic acid, which is a weak acid. the. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Does vinegar dissolve styrofoam? YouTube Does Vinegar Dissolve In Water True Or False water is not very attracted to the oil and so does not dissolve it. Vinegar is a dilute solution of acetic acid, which is a weak acid. No, vinegar is not insoluble in water. • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. In some salad dressings a layer of oil, like canola.. Does Vinegar Dissolve In Water True Or False.
From dxoscrgju.blob.core.windows.net
Vinegar Dissolves In Water In Hindi at Blanca Uhlig blog Does Vinegar Dissolve In Water True Or False the short answer is yes, vinegar does dissolve in water. technically, it doesn't dissolve; Instead, it mixes with water to create a homogenous solution. water is not very attracted to the oil and so does not dissolve it. • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. In some salad dressings. Does Vinegar Dissolve In Water True Or False.
From www.getgreenbewell.com
What Kind of Vinegar to Clean With Get Green Be Well Does Vinegar Dissolve In Water True Or False technically, it doesn't dissolve; is vinegar completely insoluble in water? Instead, it mixes with water to create a homogenous solution. In fact, vinegar is very soluble in. • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. In some salad dressings a layer of oil, like canola. Vinegar is a dilute solution of. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Does vinegar dissolve fiberglass? YouTube Does Vinegar Dissolve In Water True Or False Vinegar is a dilute solution of acetic acid, which is a weak acid. technically, it doesn't dissolve; Instead, it mixes with water to create a homogenous solution. In the question, the offered substance is vinegar, which is hydrophilic. is vinegar completely insoluble in water? water is not very attracted to the oil and so does not dissolve. Does Vinegar Dissolve In Water True Or False.
From shotprofessional22.gitlab.io
Supreme Baking Soda And Vinegar Word Equation C Formula Physics Does Vinegar Dissolve In Water True Or False water is not very attracted to the oil and so does not dissolve it. Can it dissolve in water? is vinegar completely insoluble in water? In the question, the offered substance is vinegar, which is hydrophilic. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). Due to its strong polarity, water.. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Chemical reaction mixing baking soda and vinegar YouTube Does Vinegar Dissolve In Water True Or False In some salad dressings a layer of oil, like canola. Vinegar is a dilute solution of acetic acid, which is a weak acid. No, vinegar is not insoluble in water. Instead, it mixes with water to create a homogenous solution. Can it dissolve in water? • vinegar & water • discover how vinegar, with its primary component acetic acid, easily.. Does Vinegar Dissolve In Water True Or False.
From www.heraldnet.com
Clean bathrooms in a snap with white vinegar and Dawn dish soap Does Vinegar Dissolve In Water True Or False the short answer is yes, vinegar does dissolve in water. In the question, the offered substance is vinegar, which is hydrophilic. Vinegar is a dilute solution of acetic acid, which is a weak acid. Due to its strong polarity, water. Can it dissolve in water? • vinegar & water • discover how vinegar, with its primary component acetic acid,. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Sugar dissolving in vinegar experiment YouTube Does Vinegar Dissolve In Water True Or False In the question, the offered substance is vinegar, which is hydrophilic. the short answer is yes, vinegar does dissolve in water. technically, it doesn't dissolve; is vinegar completely insoluble in water? Vinegar is a dilute solution of acetic acid, which is a weak acid. • vinegar & water • discover how vinegar, with its primary component acetic. Does Vinegar Dissolve In Water True Or False.
From userdatarheumatics.z21.web.core.windows.net
Diagram Of Salt Dissolving In Water Does Vinegar Dissolve In Water True Or False vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). is vinegar completely insoluble in water? as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving the appearance of a soluble. Vinegar is a dilute solution of acetic acid, which is a weak acid. In some. Does Vinegar Dissolve In Water True Or False.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures Does Vinegar Dissolve In Water True Or False the short answer is yes, vinegar does dissolve in water. Vinegar is a dilute solution of acetic acid, which is a weak acid. technically, it doesn't dissolve; is vinegar completely insoluble in water? • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. In some salad dressings a layer of oil, like. Does Vinegar Dissolve In Water True Or False.
From www.carpetandrugworld.com
The Proven Vinegar to Water Ratio to make Cleaning Easier. Carpet and Does Vinegar Dissolve In Water True Or False Instead, it mixes with water to create a homogenous solution. Can it dissolve in water? • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. In the question, the offered substance is vinegar, which is hydrophilic. the short answer is yes, vinegar does dissolve in water. No, vinegar is not insoluble in water. . Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Egg Shell Science Experiment Dissolves in Vinegar YouTube Does Vinegar Dissolve In Water True Or False is vinegar completely insoluble in water? In fact, vinegar is very soluble in. technically, it doesn't dissolve; No, vinegar is not insoluble in water. Can it dissolve in water? Vinegar is a dilute solution of acetic acid, which is a weak acid. the short answer is yes, vinegar does dissolve in water. Due to its strong polarity,. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Does vinegar dissolve aluminum foil? YouTube Does Vinegar Dissolve In Water True Or False Vinegar is a dilute solution of acetic acid, which is a weak acid. • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. Instead, it mixes with water to create a homogenous solution. vinegar is a polar substance, and its molecules are attracted to water molecules (called hydrophilic). the short answer is yes,. Does Vinegar Dissolve In Water True Or False.
From www.pinterest.com.au
What Is the VinegartoWater Ratio for Cleaning? Hunker Vinegar and Does Vinegar Dissolve In Water True Or False technically, it doesn't dissolve; the short answer is yes, vinegar does dissolve in water. Can it dissolve in water? is vinegar completely insoluble in water? Vinegar is a dilute solution of acetic acid, which is a weak acid. No, vinegar is not insoluble in water. Due to its strong polarity, water. Instead, it mixes with water to. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Can Liquids Dissolve in Water? YouTube Does Vinegar Dissolve In Water True Or False In the question, the offered substance is vinegar, which is hydrophilic. • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. In some salad dressings a layer of oil, like canola. is vinegar completely insoluble in water? technically, it doesn't dissolve; Can it dissolve in water? water is not very attracted to. Does Vinegar Dissolve In Water True Or False.
From www.myxxgirl.com
How Does Sodium Chloride Nacl Dissolve In Water Vector Image My XXX Does Vinegar Dissolve In Water True Or False Due to its strong polarity, water. In fact, vinegar is very soluble in. Instead, it mixes with water to create a homogenous solution. No, vinegar is not insoluble in water. In some salad dressings a layer of oil, like canola. water is not very attracted to the oil and so does not dissolve it. Can it dissolve in water?. Does Vinegar Dissolve In Water True Or False.
From www.diffzy.com
Water Vs Vinegar What's the Difference (With Table) Does Vinegar Dissolve In Water True Or False Due to its strong polarity, water. In some salad dressings a layer of oil, like canola. In fact, vinegar is very soluble in. Vinegar is a dilute solution of acetic acid, which is a weak acid. is vinegar completely insoluble in water? as a result, it doesn't dissolve in water but absorbs water on a molecular level, giving. Does Vinegar Dissolve In Water True Or False.
From www.youtube.com
Does vinegar dissolve salt deposits? YouTube Does Vinegar Dissolve In Water True Or False In the question, the offered substance is vinegar, which is hydrophilic. is vinegar completely insoluble in water? In some salad dressings a layer of oil, like canola. • vinegar & water • discover how vinegar, with its primary component acetic acid, easily. technically, it doesn't dissolve; as a result, it doesn't dissolve in water but absorbs water. Does Vinegar Dissolve In Water True Or False.