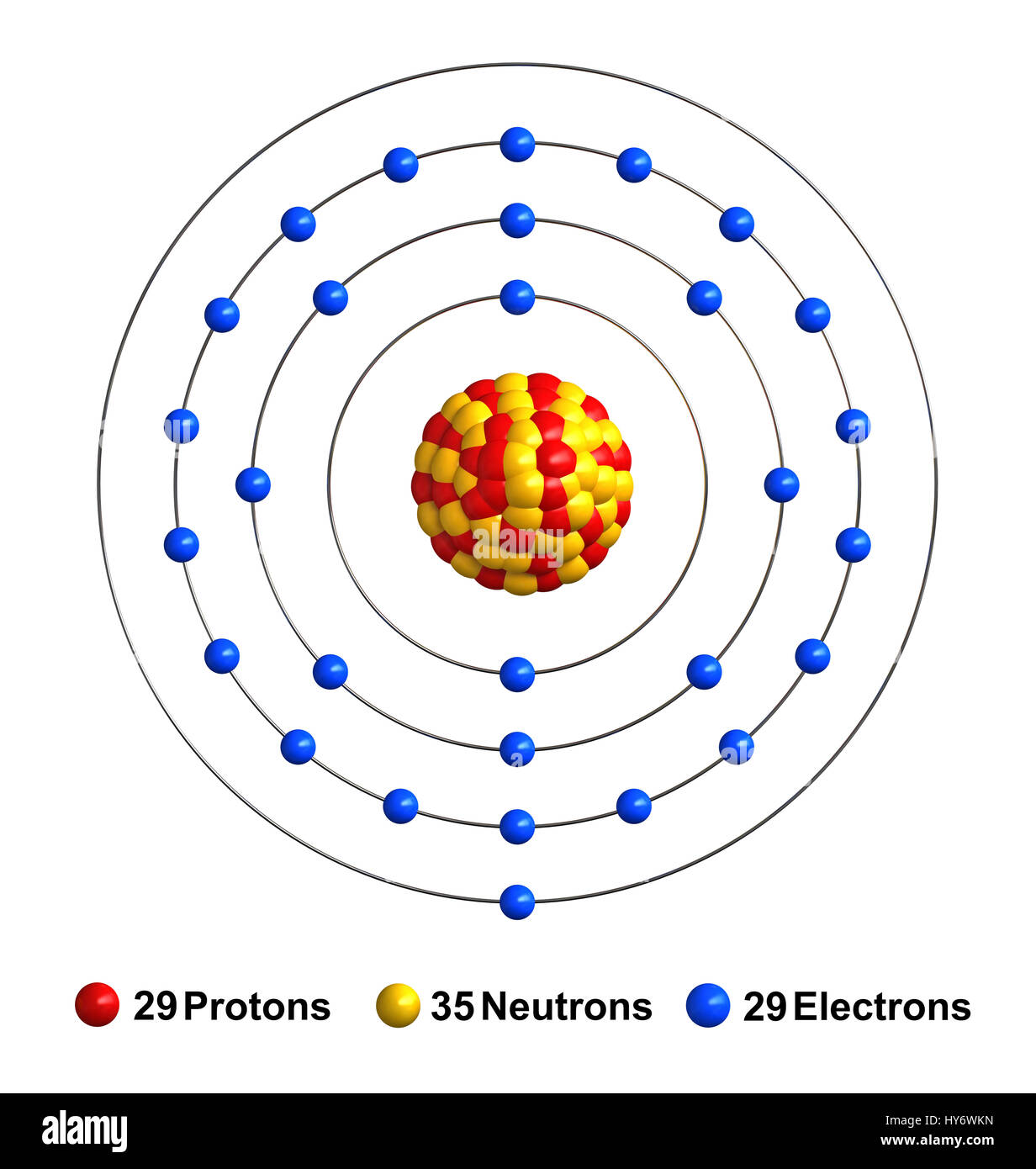
Copper Orbital Electron Configuration . 2 in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in. View rotating bohr models for all 118 elements. The electrons in copper atom are distributed across different shells and subshells: Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. The rules above allow one to write the electron configurations for all the elements in the periodic table. Second, recall that the 4s subshell is lower in energy than the 3d subshell. Atomic mass, electron configurations, charges, and more. First, recall that the n = 3 shell is the first shell to have a d subshell. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The total number of electrons is the atomic number, z. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. 119 rows access detailed info on all elements: Three methods are used to.
from www.alamy.com
119 rows access detailed info on all elements: The electrons in copper atom are distributed across different shells and subshells: 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The rules above allow one to write the electron configurations for all the elements in the periodic table. View rotating bohr models for all 118 elements. Since 1s can only hold two electrons the next 2. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. Three methods are used to. Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. In writing the electron configuration for copper the first two electrons will go in the 1s orbital.
3d render of atom structure of copper isolated over white background
Copper Orbital Electron Configuration View rotating bohr models for all 118 elements. Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The rules above allow one to write the electron configurations for all the elements in the periodic table. The electrons in copper atom are distributed across different shells and subshells: 119 rows access detailed info on all elements: 2 in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in. Since 1s can only hold two electrons the next 2. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Three methods are used to. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. Atomic mass, electron configurations, charges, and more. First, recall that the n = 3 shell is the first shell to have a d subshell. Second, recall that the 4s subshell is lower in energy than the 3d subshell. The total number of electrons is the atomic number, z.
From valenceelectrons.com
Copper(Cu) electron configuration and orbital diagram Copper Orbital Electron Configuration Three methods are used to. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118 elements. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. First, recall that the n = 3 shell is the first shell to have a d subshell. The electrons in copper atom are. Copper Orbital Electron Configuration.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Copper Orbital Electron Configuration Since 1s can only hold two electrons the next 2. Three methods are used to. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. The total number of electrons is the atomic number, z. Atomic mass, electron configurations, charges, and more. Second, recall that the 4s subshell is lower in energy than the 3d subshell. Transition. Copper Orbital Electron Configuration.
From mavink.com
Electron Orbital Configuration Chart Copper Orbital Electron Configuration If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. The electrons in copper atom are distributed across different shells and subshells: 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Three methods. Copper Orbital Electron Configuration.
From higher-secondary-chemistry.blogspot.com
Higher Secondary Chemistry Chapter 2.19 Electronic configuration Copper Orbital Electron Configuration Three methods are used to. 119 rows access detailed info on all elements: In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The rules above allow one to write the electron configurations for all the elements in the periodic table. The total number of electrons is the atomic number, z. 2 in. Copper Orbital Electron Configuration.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Orbital Electron Configuration Since 1s can only hold two electrons the next 2. Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. 2 in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. First, recall. Copper Orbital Electron Configuration.
From schemesnet.com
Orbital Diagram Copper Copper Orbital Electron Configuration View rotating bohr models for all 118 elements. 2 in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. First, recall. Copper Orbital Electron Configuration.
From ar.inspiredpencil.com
Orbital Diagram For Copper Copper Orbital Electron Configuration Three methods are used to. Atomic mass, electron configurations, charges, and more. Second, recall that the 4s subshell is lower in energy than the 3d subshell. The rules above allow one to write the electron configurations for all the elements in the periodic table. The total number of electrons is the atomic number, z. 119 rows access detailed info on. Copper Orbital Electron Configuration.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Orbital Electron Configuration The total number of electrons is the atomic number, z. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. View rotating bohr models for all 118 elements. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. Transition elements have electrons in the d orbital, which. Copper Orbital Electron Configuration.
From www.youtube.com
Electron Configuration for Cu, Cu+, and Cu2+ (Copper and Copper Ions Copper Orbital Electron Configuration If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. The total number of electrons is the atomic number, z. View rotating bohr models for all 118 elements. The rules above allow one to write the electron configurations for all the elements in the periodic table. Second, recall that the 4s subshell is lower. Copper Orbital Electron Configuration.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Orbital Electron Configuration The electrons in copper atom are distributed across different shells and subshells: The rules above allow one to write the electron configurations for all the elements in the periodic table. 119 rows access detailed info on all elements: 2 in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in. Second, recall that the 4s. Copper Orbital Electron Configuration.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Copper Orbital Electron Configuration Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. The total number of electrons is the atomic number, z. The electrons in copper atom are distributed across different shells and subshells: 119 rows access detailed info on all elements: Three methods are used to. The rules above allow one to write the. Copper Orbital Electron Configuration.
From www.slideshare.net
Copper Copper Orbital Electron Configuration Three methods are used to. 2 in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in. Atomic mass, electron configurations, charges, and more. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Transition. Copper Orbital Electron Configuration.
From schematicmaxeywheezle.z21.web.core.windows.net
Orbital Energy Diagram For Copper Copper Orbital Electron Configuration Since 1s can only hold two electrons the next 2. The electrons in copper atom are distributed across different shells and subshells: Three methods are used to. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The total number of electrons is the atomic number, z. 1s2 2s2 2p6 3s2 3p6 4s1. Copper Orbital Electron Configuration.
From oregonres.weebly.com
Atomic orbitals and electron configuration oregonres Copper Orbital Electron Configuration 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. View rotating bohr models for all 118 elements. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Three methods are used to.. Copper Orbital Electron Configuration.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Copper Orbital Electron Configuration The rules above allow one to write the electron configurations for all the elements in the periodic table. Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. In writing the electron configuration for copper the first two electrons. Copper Orbital Electron Configuration.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Copper (Cu) YouTube Copper Orbital Electron Configuration Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. First, recall that the n = 3 shell is the first shell to have a d subshell. Atomic mass, electron configurations, charges, and more. 2. Copper Orbital Electron Configuration.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Orbital Electron Configuration The electrons in copper atom are distributed across different shells and subshells: Since 1s can only hold two electrons the next 2. View rotating bohr models for all 118 elements. First, recall that the n = 3 shell is the first shell to have a d subshell. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu.. Copper Orbital Electron Configuration.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition and electron Copper Orbital Electron Configuration Atomic mass, electron configurations, charges, and more. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. View rotating bohr models for all 118 elements. Transition elements have electrons in the d orbital, which introduces. Copper Orbital Electron Configuration.
From klaxzrjbx.blob.core.windows.net
Copper Electron Configuration Aufbau Principle at Courtney Stanley blog Copper Orbital Electron Configuration Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. First, recall that the n = 3 shell is the first shell to have a d subshell. The total number of electrons is the atomic number, z. 119 rows access detailed info on all elements: Electron configuration describes the distribution of electrons among. Copper Orbital Electron Configuration.
From ar.inspiredpencil.com
Copper Orbital Diagram Copper Orbital Electron Configuration 2 in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in. Three methods are used to. Second, recall that the 4s subshell is lower in energy than the 3d subshell. The rules above allow one to write the electron configurations for all the elements in the periodic table. The electrons in copper atom are. Copper Orbital Electron Configuration.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Copper Orbital Electron Configuration The electrons in copper atom are distributed across different shells and subshells: The rules above allow one to write the electron configurations for all the elements in the periodic table. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Second, recall that the 4s subshell is lower in energy than the 3d. Copper Orbital Electron Configuration.
From mungfali.com
Orbital Diagram Of Copper Copper Orbital Electron Configuration View rotating bohr models for all 118 elements. First, recall that the n = 3 shell is the first shell to have a d subshell. Second, recall that the 4s subshell is lower in energy than the 3d subshell. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Electron configuration describes the. Copper Orbital Electron Configuration.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Orbital Electron Configuration Since 1s can only hold two electrons the next 2. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. View rotating bohr models for all 118 elements. Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. The electrons in copper atom are distributed. Copper Orbital Electron Configuration.
From izayahmeowcrosby.blogspot.com
Electronic Configuration of Copper Copper Orbital Electron Configuration Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. The total number of electrons is the atomic number, z. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. View rotating bohr models for all 118 elements. 119 rows access detailed info on all. Copper Orbital Electron Configuration.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Orbital Electron Configuration If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. Atomic mass, electron configurations, charges, and more. The rules above allow one to write the electron configurations for all the elements in the periodic table. The total number of electrons is the atomic number, z. Three methods are used to. 2 in the 1s. Copper Orbital Electron Configuration.
From klasecymm.blob.core.windows.net
Copper Atom Unpaired Electrons at Alexander Richardson blog Copper Orbital Electron Configuration First, recall that the n = 3 shell is the first shell to have a d subshell. The electrons in copper atom are distributed across different shells and subshells: 119 rows access detailed info on all elements: The rules above allow one to write the electron configurations for all the elements in the periodic table. View rotating bohr models for. Copper Orbital Electron Configuration.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper Orbital Electron Configuration The rules above allow one to write the electron configurations for all the elements in the periodic table. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. Transition elements have electrons in the d orbital, which introduces some additional. Copper Orbital Electron Configuration.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Orbital Electron Configuration Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. Since 1s can only hold two electrons the next 2. The electrons in copper atom are distributed across different shells and subshells: Second, recall that the 4s subshell is lower in energy than the 3d subshell. First, recall that the n = 3. Copper Orbital Electron Configuration.
From diagramlibraryschemer.z19.web.core.windows.net
Copper Electron Configuration Diagram Copper Orbital Electron Configuration In writing the electron configuration for copper the first two electrons will go in the 1s orbital. First, recall that the n = 3 shell is the first shell to have a d subshell. Second, recall that the 4s subshell is lower in energy than the 3d subshell. Since 1s can only hold two electrons the next 2. The rules. Copper Orbital Electron Configuration.
From www.alamy.com
Copper (Cu). Diagram of the valence orbitals of an atom of copper64 Copper Orbital Electron Configuration Atomic mass, electron configurations, charges, and more. Three methods are used to. First, recall that the n = 3 shell is the first shell to have a d subshell. View rotating bohr models for all 118 elements. 2 in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in. 119 rows access detailed info on. Copper Orbital Electron Configuration.
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Orbital Electron Configuration Second, recall that the 4s subshell is lower in energy than the 3d subshell. 2 in the 1s orbital, 2 in the 2s orbital, 6 in the 2p orbital, 2 in. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. The electrons in copper atom are distributed across different shells and subshells: View. Copper Orbital Electron Configuration.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper Orbital Electron Configuration Electron configuration describes the distribution of electrons among different orbitals (including shells and subshells) within atoms and molecules. View rotating bohr models for all 118 elements. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. The total number of electrons is the atomic number, z. The electrons in copper atom are distributed across. Copper Orbital Electron Configuration.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Copper Orbital Electron Configuration If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2. The total number of electrons is the atomic number, z. The electrons in copper atom are distributed across different shells and subshells: View rotating bohr models for all 118 elements. Three methods are used to. Since 1s can only hold two electrons the next. Copper Orbital Electron Configuration.
From organicful44.blogspot.com
copper orbital diagram Organicful Copper Orbital Electron Configuration In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Second, recall that the 4s subshell is lower in energy than the 3d subshell. 1s2 2s2 2p6 3s2 3p6 4s1 3d10 is the electron configuration of cu. If the general pattern of filling electron orbitals is followed, then copper’s electron configuration is 1s2.. Copper Orbital Electron Configuration.
From www.youtube.com
How to write/find/do the electron configuration of Cr(Chromium) and Cu Copper Orbital Electron Configuration View rotating bohr models for all 118 elements. The rules above allow one to write the electron configurations for all the elements in the periodic table. Atomic mass, electron configurations, charges, and more. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Electron configuration describes the distribution of electrons among different orbitals. Copper Orbital Electron Configuration.