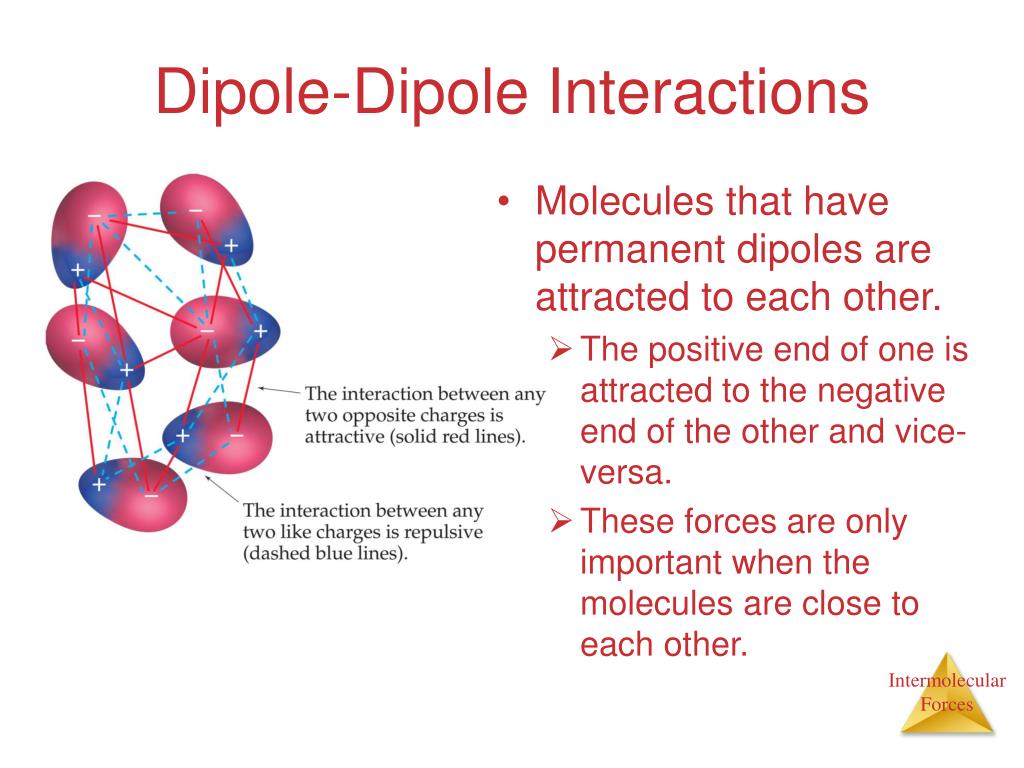
Point Dipole Definition . (in this context, “close” means that the distance d. The electric field is inversely proportional. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. Alert chemists typically define the dipole moment as pointing in the opposite direction. Two equal and opposite charges that are “close” to each other. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → = p → is held constant. The direction of the dipole moment is that it points from the negative charge to the positive charge. Earlier we discussed, and calculated, the electric field of a dipole:
from www.slideserve.com
The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → = p → is held constant. The electric field is inversely proportional. Two equal and opposite charges that are “close” to each other. (in this context, “close” means that the distance d. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). Earlier we discussed, and calculated, the electric field of a dipole: Alert chemists typically define the dipole moment as pointing in the opposite direction. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. The direction of the dipole moment is that it points from the negative charge to the positive charge.
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint
Point Dipole Definition A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. The direction of the dipole moment is that it points from the negative charge to the positive charge. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). Alert chemists typically define the dipole moment as pointing in the opposite direction. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. Two equal and opposite charges that are “close” to each other. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → = p → is held constant. Earlier we discussed, and calculated, the electric field of a dipole: The electric field is inversely proportional. (in this context, “close” means that the distance d.
From www.amateur-radio-wiki.net
Learn What a Dipole Is in Physics and Chemistry Point Dipole Definition Two equal and opposite charges that are “close” to each other. The electric field is inversely proportional. (in this context, “close” means that the distance d. Alert chemists typically define the dipole moment as pointing in the opposite direction. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated. Point Dipole Definition.
From lo-overview.blogspot.com
O V E R V I E W Point Dipole Definition The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → = p → is held constant. Earlier we discussed, and calculated, the electric field of a dipole: A point dipole is an idealized. Point Dipole Definition.
From collegedunia.com
Electric Dipole Definition, Significance and Electric Dipole Moment Point Dipole Definition Two equal and opposite charges that are “close” to each other. The electric field is inversely proportional. The direction of the dipole moment is that it points from the negative charge to the positive charge. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the. Point Dipole Definition.
From study.com
Hydrogen Bonding, DipoleDipole & IonDipole Forces Strong Point Dipole Definition (in this context, “close” means that the distance d. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). Two equal and opposite charges that are “close” to each other. The point dipole is defined as the limit of two equal and opposite charges. Point Dipole Definition.
From diagramlibrarytwit.z5.web.core.windows.net
Electric Dipole Diagram Physics 2 Point Dipole Definition A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. Earlier we discussed, and calculated, the electric field of a dipole: The direction of the dipole moment is that it points from the negative charge to the positive charge. The electric field is inversely proportional. Alert chemists typically define. Point Dipole Definition.
From www.doubtnut.com
A point dipole with an electric moment p oriented in the positive Point Dipole Definition An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. The point dipole is defined as the limit of two equal and opposite. Point Dipole Definition.
From www.geeksforgeeks.org
Electric Dipole Definition, Formula, Units, Examples, and FAQs Point Dipole Definition Earlier we discussed, and calculated, the electric field of a dipole: The electric field is inversely proportional. Alert chemists typically define the dipole moment as pointing in the opposite direction. (in this context, “close” means that the distance d. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance.. Point Dipole Definition.
From www.slideserve.com
PPT DipoleDipole PowerPoint Presentation, free download ID6900668 Point Dipole Definition The electric field is inversely proportional. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition. Point Dipole Definition.
From schematicdopatgety.z22.web.core.windows.net
Electric Dipole Diagram Physics 2 Point Dipole Definition (in this context, “close” means that the distance d. Two equal and opposite charges that are “close” to each other. Earlier we discussed, and calculated, the electric field of a dipole: An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). The direction of. Point Dipole Definition.
From wiringdbfilicides.z19.web.core.windows.net
How To Determine Dipole Dipole Point Dipole Definition A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → = p. Point Dipole Definition.
From schematicpartduo.z4.web.core.windows.net
Diagram Of An Electric Dipole Point Dipole Definition A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. The direction of the dipole moment is that it points from the negative charge to the positive charge. The electric field is inversely proportional. Earlier we discussed, and calculated, the electric field of a dipole: An electric dipole is. Point Dipole Definition.
From www.pinterest.com
Dipole Easy Science Teaching chemistry, Chemistry education Point Dipole Definition Two equal and opposite charges that are “close” to each other. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → = p → is held constant. The direction of the dipole moment. Point Dipole Definition.
From www.alamy.com
Field lines of a point dipole. Pole of a physical dipole of any type Point Dipole Definition The electric field is inversely proportional. (in this context, “close” means that the distance d. Earlier we discussed, and calculated, the electric field of a dipole: The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that. Point Dipole Definition.
From www.slideserve.com
PPT Chapter 10 Intermolecular Forces, Liquids, and Solids PowerPoint Point Dipole Definition The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → = p → is held constant. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by. Point Dipole Definition.
From www.researchgate.net
Dipoledipole configuration, including electric field lines (solid) and Point Dipole Definition Two equal and opposite charges that are “close” to each other. Earlier we discussed, and calculated, the electric field of a dipole: (in this context, “close” means that the distance d. Alert chemists typically define the dipole moment as pointing in the opposite direction. An electric dipole is made up of two point charges that have equal but opposite electric. Point Dipole Definition.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint Point Dipole Definition Earlier we discussed, and calculated, the electric field of a dipole: The electric field is inversely proportional. Two equal and opposite charges that are “close” to each other. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). The point dipole is defined as. Point Dipole Definition.
From www.youtube.com
Ion Dipole Forces & Ion Induced Dipole Interactions Chemistry YouTube Point Dipole Definition Two equal and opposite charges that are “close” to each other. The electric field is inversely proportional. Alert chemists typically define the dipole moment as pointing in the opposite direction. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. An electric dipole is made up of two point. Point Dipole Definition.
From www.chemistrysteps.com
Molecular Dipole The Overall Polarity of the Molecule Chemistry Steps Point Dipole Definition The electric field is inversely proportional. Alert chemists typically define the dipole moment as pointing in the opposite direction. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. Two equal and opposite charges that are “close” to each other. The direction of the dipole moment is that it. Point Dipole Definition.
From www.crediblehulk.org
DipoleDipole Interactions The Credible Hulk Point Dipole Definition The direction of the dipole moment is that it points from the negative charge to the positive charge. Earlier we discussed, and calculated, the electric field of a dipole: (in this context, “close” means that the distance d. The electric field is inversely proportional. Two equal and opposite charges that are “close” to each other. Alert chemists typically define the. Point Dipole Definition.
From www.wou.edu
CH103 Chapter 5 Covalent Bonds and Introduction to Organic Molecules Point Dipole Definition The direction of the dipole moment is that it points from the negative charge to the positive charge. (in this context, “close” means that the distance d. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. Two equal and opposite charges that are “close” to each other. Alert. Point Dipole Definition.
From www.researchgate.net
Pointdipole modeling. a, Schematic of the pointdipole model. a, z Point Dipole Definition A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. Two equal and opposite charges that are “close” to each other. Alert chemists typically define the dipole moment as pointing in the opposite direction. The direction of the dipole moment is that it points from the negative charge to. Point Dipole Definition.
From devinitionva.blogspot.com
Definition Of Dipole In Chemistry DEFINITIONVA Point Dipole Definition Two equal and opposite charges that are “close” to each other. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → = p → is held constant. Earlier we discussed, and calculated, the. Point Dipole Definition.
From www.expii.com
IonDipole Forces — Definition & Overview Expii Point Dipole Definition Earlier we discussed, and calculated, the electric field of a dipole: Two equal and opposite charges that are “close” to each other. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → =. Point Dipole Definition.
From www.aakash.ac.in
Dipole Electric Field Formula, Definition & Examples AESL Point Dipole Definition A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. The direction of the dipole moment is that it points from the negative charge to the positive charge. Two equal and opposite charges that are “close” to each other. (in this context, “close” means that the distance d. The. Point Dipole Definition.
From www.researchgate.net
Point dipole in rectilinear motion along a straight path. Download Point Dipole Definition (in this context, “close” means that the distance d. The direction of the dipole moment is that it points from the negative charge to the positive charge. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). The point dipole is defined as the. Point Dipole Definition.
From www.chemistrylearner.com
Iondipole Forces (Interaction) Definition and Examples Point Dipole Definition The direction of the dipole moment is that it points from the negative charge to the positive charge. (in this context, “close” means that the distance d. The electric field is inversely proportional. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ. Point Dipole Definition.
From study.com
Dipole & Dipole Moment What is Molecular Polarity? Lesson Point Dipole Definition The direction of the dipole moment is that it points from the negative charge to the positive charge. Two equal and opposite charges that are “close” to each other. A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. Earlier we discussed, and calculated, the electric field of a. Point Dipole Definition.
From manualenginesantos.z19.web.core.windows.net
How To Determine Dipole Dipole Point Dipole Definition Alert chemists typically define the dipole moment as pointing in the opposite direction. The direction of the dipole moment is that it points from the negative charge to the positive charge. (in this context, “close” means that the distance d. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are. Point Dipole Definition.
From www.pinterest.ph
Dipole Dipole Force Easy Science 10th grade science, Organic Point Dipole Definition Two equal and opposite charges that are “close” to each other. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition that q δ → = p → is held constant. Earlier we discussed, and calculated, the. Point Dipole Definition.
From www.slideserve.com
PPT MIT Class Particle Interactions Coulomb’s Law PowerPoint Point Dipole Definition The direction of the dipole moment is that it points from the negative charge to the positive charge. (in this context, “close” means that the distance d. The point dipole is defined as the limit of two equal and opposite charges ± q, separated by a small displacement δ →, in the limit δ → → 0 under the condition. Point Dipole Definition.
From chem.libretexts.org
Dipole Moments Chemistry LibreTexts Point Dipole Definition Two equal and opposite charges that are “close” to each other. (in this context, “close” means that the distance d. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). Earlier we discussed, and calculated, the electric field of a dipole: The electric field. Point Dipole Definition.
From www.researchgate.net
Point dipole in rectilinear motion along a straight path. Download Point Dipole Definition A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. The electric field is inversely proportional. Alert chemists typically define the dipole moment as pointing in the opposite direction. The direction of the dipole moment is that it points from the negative charge to the positive charge. Two equal. Point Dipole Definition.
From brilliant.org
Dipole Interactions Brilliant Math & Science Wiki Point Dipole Definition The electric field is inversely proportional. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). The direction of the dipole moment is that it points from the negative charge to the positive charge. (in this context, “close” means that the distance d. Two. Point Dipole Definition.
From chemistnotes.com
Dipole moment Definition, Formula, & Examples Chemistry Notes Point Dipole Definition Two equal and opposite charges that are “close” to each other. (in this context, “close” means that the distance d. The electric field is inversely proportional. An electric dipole is made up of two point charges that have equal but opposite electric charges (q) and are separated by a short distance (d). Earlier we discussed, and calculated, the electric field. Point Dipole Definition.
From www.mindomo.com
Unit 1 Mind Map Point Dipole Definition A point dipole is an idealized electric dipole consisting of two equal and opposite charges separated by an infinitesimally small distance. (in this context, “close” means that the distance d. Alert chemists typically define the dipole moment as pointing in the opposite direction. The point dipole is defined as the limit of two equal and opposite charges ± q, separated. Point Dipole Definition.