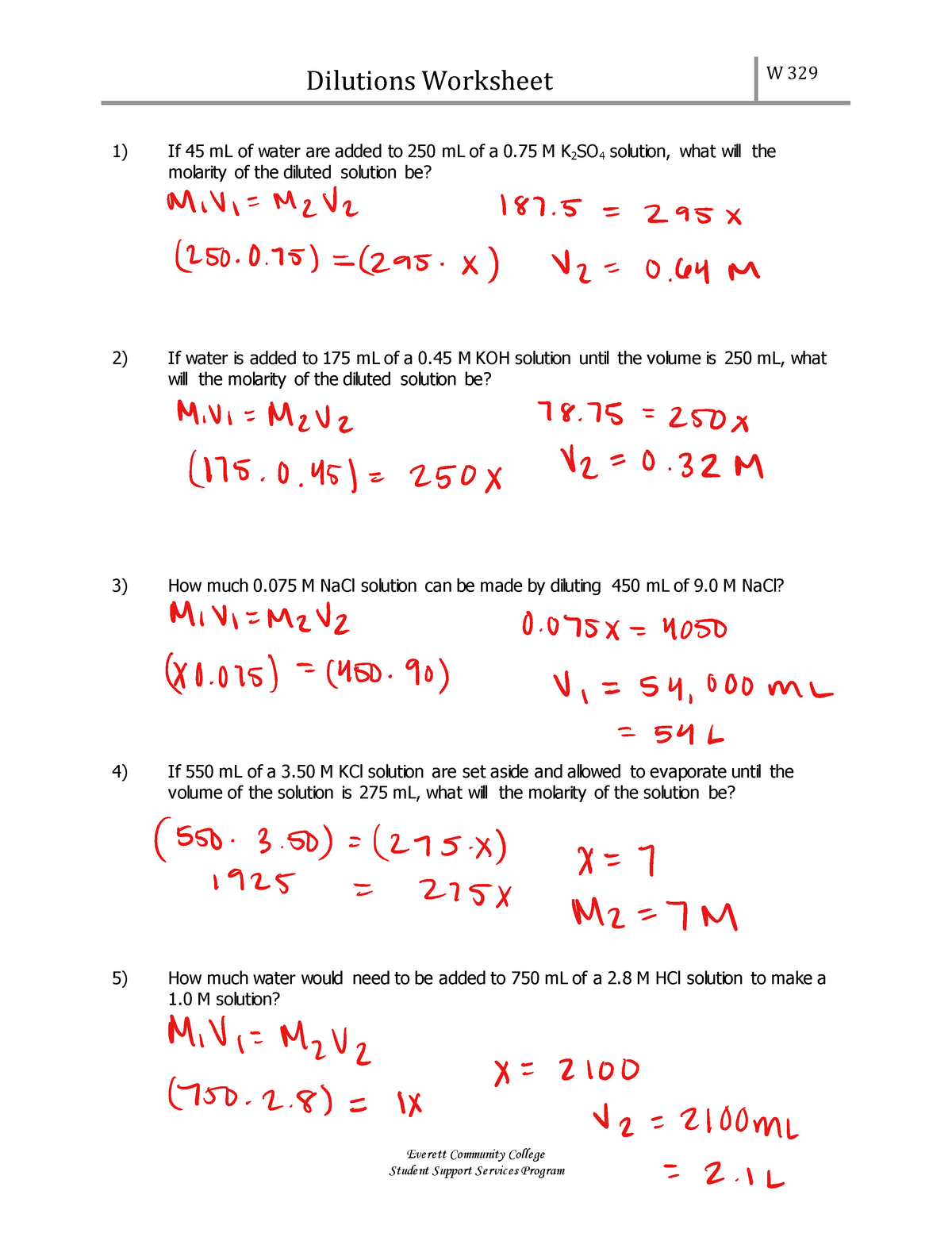
Dilution Chem Worksheet 15-5 Answers . 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. Concentration is the removal of solvent, which. a solution can be made less concentrated in a process called dilution. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. This is accomplished by adding more solvent. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. Worksheets are dilutions work, dilutions work w 329, dilution name chem.
from www.studocu.com
dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. Concentration is the removal of solvent, which. a solution can be made less concentrated in a process called dilution. This is accomplished by adding more solvent. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?
Dilution Worksheet eskandari summer Dilutions Worksheet W 329
Dilution Chem Worksheet 15-5 Answers Worksheets are dilutions work, dilutions work w 329, dilution name chem. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. a solution can be made less concentrated in a process called dilution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Worksheets are dilutions work, dilutions work w 329, dilution name chem. Concentration is the removal of solvent, which. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. This is accomplished by adding more solvent. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing.
From www.scribd.com
Dilutions Worksheet Key For Chemistry PDF Dilution Chem Worksheet 15-5 Answers Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Worksheets are dilutions work, dilutions work w 329, dilution name chem. 1) if i have 340 ml of a 0.5 m. Dilution Chem Worksheet 15-5 Answers.
From www.scribd.com
Making Dilutions Worksheet PDF Chemistry Physical Sciences Dilution Chem Worksheet 15-5 Answers How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? Concentration is the removal of solvent, which. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. 1) if i have 340 ml of a 0.5. Dilution Chem Worksheet 15-5 Answers.
From studyzonepawlowski.z22.web.core.windows.net
Solubility Curve Practice Problems Worksheets Answers Dilution Chem Worksheet 15-5 Answers This is accomplished by adding more solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. How much of a 15.0 m stock solution do you need to. Dilution Chem Worksheet 15-5 Answers.
From www.studocu.com
Grade 11 Chemistry Dilution Worksheet © John Erickson, 2005 WS15 Dilution Chem Worksheet 15-5 Answers 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Concentration is. Dilution Chem Worksheet 15-5 Answers.
From thekidsworksheet.com
Dilutions Worksheet Answer Key Thekidsworksheet Dilution Chem Worksheet 15-5 Answers This is accomplished by adding more solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Worksheets are dilutions work, dilutions work w 329, dilution name chem. dilution. Dilution Chem Worksheet 15-5 Answers.
From excelkayra.us
Dilutions Worksheet Answers Kayra Excel Dilution Chem Worksheet 15-5 Answers dilution is the addition of solvent, which decreases the concentration of the solute in the solution. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. This is accomplished by adding more solvent. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Add more solvent to. Dilution Chem Worksheet 15-5 Answers.
From thekidsworksheet.com
Solutions And Dilutions Worksheet Thekidsworksheet Dilution Chem Worksheet 15-5 Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. Worksheets are dilutions work, dilutions work w 329, dilution name chem. a solution can be made less concentrated in a process called. Dilution Chem Worksheet 15-5 Answers.
From thekidsworksheet.com
Dilutions Worksheet Answer Key Thekidsworksheet Dilution Chem Worksheet 15-5 Answers Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity. Dilution Chem Worksheet 15-5 Answers.
From studylib.net
Dilutions Worksheet 11UChem Dilution Chem Worksheet 15-5 Answers Worksheets are dilutions work, dilutions work w 329, dilution name chem. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? 1) if i add 25 ml of water to 125. Dilution Chem Worksheet 15-5 Answers.
From www.formsbank.com
Dilution Worksheet With Answers printable pdf download Dilution Chem Worksheet 15-5 Answers This is accomplished by adding more solvent. Concentration is the removal of solvent, which. Worksheets are dilutions work, dilutions work w 329, dilution name chem. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. for questions 1. Dilution Chem Worksheet 15-5 Answers.
From studylib.net
Dilutions Worksheet Dilution Chem Worksheet 15-5 Answers Worksheets are dilutions work, dilutions work w 329, dilution name chem. This is accomplished by adding more solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m. Dilution Chem Worksheet 15-5 Answers.
From studylib.net
Dilution Dilution Chem Worksheet 15-5 Answers Worksheets are dilutions work, dilutions work w 329, dilution name chem. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution,. Dilution Chem Worksheet 15-5 Answers.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Dilution Chem Worksheet 15-5 Answers Worksheets are dilutions work, dilutions work w 329, dilution name chem. Concentration is the removal of solvent, which. This is accomplished by adding more solvent. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity. Dilution Chem Worksheet 15-5 Answers.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Dilution Chem Worksheet 15-5 Answers Worksheets are dilutions work, dilutions work w 329, dilution name chem. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. This is accomplished by adding more. Dilution Chem Worksheet 15-5 Answers.
From igneousrocksworksheetanswers.blogspot.com
Dilutions Worksheet Answers Maths Printables Free Dilution Chem Worksheet 15-5 Answers dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. This is accomplished by adding more solvent. Concentration is the removal of solvent, which. Worksheets are dilutions work, dilutions work w 329, dilution name chem. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. Dilution Chem Worksheet 15-5 Answers.
From gambr.co
️Dilutions Worksheet 1 Answers Free Download Gambr.co Dilution Chem Worksheet 15-5 Answers for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. Worksheets are dilutions work, dilutions work w 329, dilution name chem. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. dilution problems worksheet (m. Dilution Chem Worksheet 15-5 Answers.
From www.docsity.com
Molarity and Dilutions Practice Worksheet Key Docsity Dilution Chem Worksheet 15-5 Answers 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity. Dilution Chem Worksheet 15-5 Answers.
From printabledeancraigxqn4.z22.web.core.windows.net
Identifying Types Of Chemical Reactions Worksheets Answer Ke Dilution Chem Worksheet 15-5 Answers Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. This is accomplished by adding more solvent. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. a solution can be made less concentrated in a process called dilution. 1) if i add. Dilution Chem Worksheet 15-5 Answers.
From igneousrocksworksheetanswers.blogspot.com
Dilutions Worksheet Answers Maths Printables Free Dilution Chem Worksheet 15-5 Answers Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. a. Dilution Chem Worksheet 15-5 Answers.
From learningzoneschreiber.z21.web.core.windows.net
Types Of Chemical Reactions Worksheet With Answers Dilution Chem Worksheet 15-5 Answers dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. This is accomplished. Dilution Chem Worksheet 15-5 Answers.
From studylib.net
Dilution Worksheet Dilution Chem Worksheet 15-5 Answers dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. Worksheets are. Dilution Chem Worksheet 15-5 Answers.
From www.studocu.com
Dilution Worksheet eskandari summer Dilutions Worksheet W 329 Dilution Chem Worksheet 15-5 Answers Worksheets are dilutions work, dilutions work w 329, dilution name chem. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. a solution can be made less concentrated in a process called dilution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. 1). Dilution Chem Worksheet 15-5 Answers.
From thekidsworksheet.com
Dilutions Worksheet Answers Thekidsworksheet Dilution Chem Worksheet 15-5 Answers dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. Worksheets are dilutions work, dilutions work w 329, dilution name chem.. Dilution Chem Worksheet 15-5 Answers.
From www.studocu.com
Dilutions Worksheet Dilution problems CHEM 1010 Studocu Dilution Chem Worksheet 15-5 Answers This is accomplished by adding more solvent. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution?. Dilution Chem Worksheet 15-5 Answers.
From kidsworksheetfun.com
Serial Dilutions Worksheet Answers Kidsworksheetfun Dilution Chem Worksheet 15-5 Answers Worksheets are dilutions work, dilutions work w 329, dilution name chem. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. This is accomplished by adding more solvent. a solution can be made less concentrated in a process called dilution. Add more solvent to a solution, thus volume of solution increases while moles of solute. Dilution Chem Worksheet 15-5 Answers.
From www.pinterest.com
Molarity and Dilutions Notes and Worksheet Set Molarity equation Dilution Chem Worksheet 15-5 Answers Worksheets are dilutions work, dilutions work w 329, dilution name chem. Concentration is the removal of solvent, which. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. Add more solvent to a solution, thus volume of solution increases while moles of solute remains. Dilution Chem Worksheet 15-5 Answers.
From thekidsworksheet.com
Dilutions Worksheet Microbiology Thekidsworksheet Dilution Chem Worksheet 15-5 Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. dilution problems worksheet (m 1 v 1 = m 2 v 2) 1. dilution is the addition. Dilution Chem Worksheet 15-5 Answers.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Chem Worksheet 15-5 Answers for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant,. Dilution Chem Worksheet 15-5 Answers.
From thekidsworksheet.com
Making Solutions And Dilutions Worksheet Answers Thekidsworksheet Dilution Chem Worksheet 15-5 Answers How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. 1) if i add. Dilution Chem Worksheet 15-5 Answers.
From www.docsity.com
Making Dilutions Worksheet Answers Key Docsity Dilution Chem Worksheet 15-5 Answers for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant,. Dilution Chem Worksheet 15-5 Answers.
From martinlindelof.com
Dilution Practice Problems Worksheet Answers Dilution Chem Worksheet 15-5 Answers for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. This is accomplished by adding more solvent. Concentration is the removal of solvent, which. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilution. Dilution Chem Worksheet 15-5 Answers.
From www.chemistryworksheet.com
Dilution Calculation Worksheet With Answers Printable Pdf Download Dilution Chem Worksheet 15-5 Answers How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? This is accomplished by adding more solvent. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 1) if i add 25 ml of water to 125 ml of a 0.15. Dilution Chem Worksheet 15-5 Answers.
From www.chemistryworksheet.com
Dilution Problems Chemistry Worksheet With Answers Dilution Chem Worksheet 15-5 Answers 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. for questions 1 and 2, the units for your final answer should be “m”, or “molar”, because you’re trying to find the molarity of the. Concentration is the removal of solvent, which. How much of a. Dilution Chem Worksheet 15-5 Answers.
From studylib.net
Solutions & Dilutions Worksheet Dilution Chem Worksheet 15-5 Answers 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. How much of a 15.0 m stock solution do you need to prepare 250 ml of a 2.35 m hf solution? dilution is the addition of solvent, which decreases the concentration of the solute in the solution. 1) if i add. Dilution Chem Worksheet 15-5 Answers.
From worksheetideasbylinda.netlify.app
Molarity By Dilution Worksheet Dilution Chem Worksheet 15-5 Answers a solution can be made less concentrated in a process called dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the. Add more solvent to a solution, thus volume of solution increases while moles of solute remains constant, causing. Worksheets are dilutions work, dilutions work. Dilution Chem Worksheet 15-5 Answers.