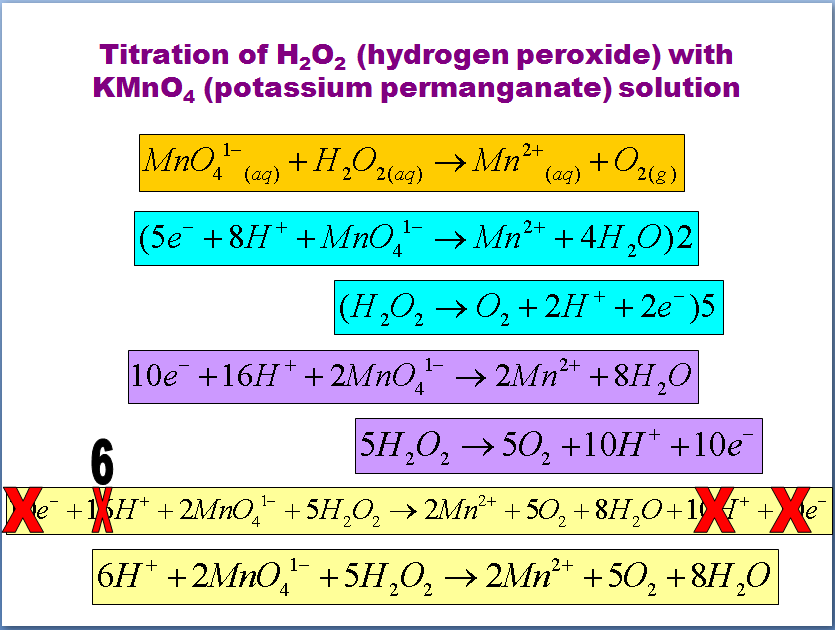
Hydrogen Peroxide In Redox Titration . what you need to know. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. Potassium permanganate acts as an oxidizing agent, oxidizing. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium.
from hhsapchemistrybrueckner201112.blogspot.com
hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. what you need to know. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. Potassium permanganate acts as an oxidizing agent, oxidizing.
Heritage High School Mr. Brueckner's AP Chemistry Class 201112
Hydrogen Peroxide In Redox Titration redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. what you need to know. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. Potassium permanganate acts as an oxidizing agent, oxidizing. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. To determine the concentration of hydrogen peroxide in solution, the method called manganometry.
From www.youtube.com
Hydrogen Peroxide with Potassium PermanganateTitrationRedox Reaction Hydrogen Peroxide In Redox Titration redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. Potassium permanganate acts as an oxidizing agent, oxidizing. hydrogen peroxide in a diluted. Hydrogen Peroxide In Redox Titration.
From hhsapchemistrybrueckner201112.blogspot.com
Heritage High School Mr. Brueckner's AP Chemistry Class 201112 Hydrogen Peroxide In Redox Titration Potassium permanganate acts as an oxidizing agent, oxidizing. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. what you need to know. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. To determine the concentration. Hydrogen Peroxide In Redox Titration.
From childhealthpolicy.vumc.org
⭐ Determination of peroxide value by titration. Using Redox Titration Hydrogen Peroxide In Redox Titration what you need to know. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. Potassium permanganate acts as an oxidizing agent, oxidizing. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. hydrogen peroxide in a diluted portion of the. Hydrogen Peroxide In Redox Titration.
From www.slideserve.com
PPT Analysis of Hydrogen Peroxide (redox titration) PowerPoint Hydrogen Peroxide In Redox Titration hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Potassium permanganate acts as. Hydrogen Peroxide In Redox Titration.
From studylib.net
Name Redox titration of Hydrogen Peroxide 5H2O2 + 2KMnO4 + 3H Hydrogen Peroxide In Redox Titration what you need to know. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. Potassium permanganate acts as an oxidizing agent, oxidizing. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96. Hydrogen Peroxide In Redox Titration.
From childhealthpolicy.vumc.org
⭐ Determination of peroxide value by titration. Using Redox Titration Hydrogen Peroxide In Redox Titration hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. Potassium permanganate acts as an oxidizing agent, oxidizing. . Hydrogen Peroxide In Redox Titration.
From es.scribd.com
Hydrogen Peroxide Analysis by FlinnSci Redox Titration Hydrogen Peroxide In Redox Titration we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. what you need to know. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. redox titration is a laboratory method of determining the concentration of. Hydrogen Peroxide In Redox Titration.
From stepmomdialogues.blogspot.com
Determining The Concentration Of Hydrogen Peroxide By Redox Titration Hydrogen Peroxide In Redox Titration hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. Potassium permanganate acts as an oxidizing agent, oxidizing. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. we present the determination. Hydrogen Peroxide In Redox Titration.
From www.numerade.com
SOLVED Redox titrations are used to determine the amounts of oxidizing Hydrogen Peroxide In Redox Titration redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. what you need to know. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an. Hydrogen Peroxide In Redox Titration.
From www.numerade.com
SOLVED The concentration of hydrogen peroxide solution can be found by Hydrogen Peroxide In Redox Titration what you need to know. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. . Hydrogen Peroxide In Redox Titration.
From www.youtube.com
Redox Reaction 10, Double Titration and Hydrogen Peroxide YouTube Hydrogen Peroxide In Redox Titration redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the. Hydrogen Peroxide In Redox Titration.
From www.slideserve.com
PPT Balancing Redox Equations in Acidic Conditions PowerPoint Hydrogen Peroxide In Redox Titration we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. Potassium permanganate acts as an oxidizing agent, oxidizing. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. hydrogen peroxide in a diluted portion of the sample is quantitatively. Hydrogen Peroxide In Redox Titration.
From www.studocu.com
Lab I1 2425174 Redox Titration of Hydrogen Peroxide CHEM2001 Hydrogen Peroxide In Redox Titration we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. what you need to know. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. . Hydrogen Peroxide In Redox Titration.
From www.slideserve.com
PPT Analysis of Hydrogen Peroxide (redox titration) PowerPoint Hydrogen Peroxide In Redox Titration hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. what you need to know. Potassium permanganate acts as an oxidizing agent,. Hydrogen Peroxide In Redox Titration.
From childhealthpolicy.vumc.org
⭐ Determination of peroxide value by titration. Using Redox Titration Hydrogen Peroxide In Redox Titration redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. hydrogen peroxide (h 2 o 2). Hydrogen Peroxide In Redox Titration.
From www.youtube.com
Analysis of Hydrogen Peroxide Redox Titration YouTube Hydrogen Peroxide In Redox Titration redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. what you need to know. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with. Hydrogen Peroxide In Redox Titration.
From www.youtube.com
AP Chemistry Investigation 8 Redox Titration of Hydrogen Peroxide Hydrogen Peroxide In Redox Titration To determine the concentration of hydrogen peroxide in solution, the method called manganometry. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. hydrogen peroxide (h 2. Hydrogen Peroxide In Redox Titration.
From www.slideserve.com
PPT Analysis of Hydrogen Peroxide (redox titration) PowerPoint Hydrogen Peroxide In Redox Titration hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. Potassium permanganate acts as an oxidizing agent, oxidizing. what you need to know. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. redox titration is. Hydrogen Peroxide In Redox Titration.
From stepmomdialogues.blogspot.com
Determining The Concentration Of Hydrogen Peroxide By Redox Titration Hydrogen Peroxide In Redox Titration To determine the concentration of hydrogen peroxide in solution, the method called manganometry. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. what you need to know. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. we present. Hydrogen Peroxide In Redox Titration.
From www.studocu.com
Hydrogen Peroxide Assay ASSAY OF HYDROGEN PEROXIDE BY. REDUCTION Hydrogen Peroxide In Redox Titration Potassium permanganate acts as an oxidizing agent, oxidizing. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. To determine the concentration of hydrogen peroxide in solution, the method called. Hydrogen Peroxide In Redox Titration.
From www.youtube.com
AP Chemistry Investigation 8 Redox Titration of Hydrogen Peroxide by Hydrogen Peroxide In Redox Titration hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. hydrogen peroxide in a diluted portion of the. Hydrogen Peroxide In Redox Titration.
From www.youtube.com
How to Determine the Volume Strength of Hydrogen peroxide? Redox Hydrogen Peroxide In Redox Titration what you need to know. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c. Hydrogen Peroxide In Redox Titration.
From www.vrogue.co
Types Of Titration Acid Base Titrations Redox Amp Com vrogue.co Hydrogen Peroxide In Redox Titration redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. what you need to know. hydrogen peroxide (h 2 o 2) is. Hydrogen Peroxide In Redox Titration.
From www.chemicalslearning.com
Hydrogen Peroxide Assay by Titration Hydrogen Peroxide Testing Method Hydrogen Peroxide In Redox Titration Potassium permanganate acts as an oxidizing agent, oxidizing. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. we present the determination. Hydrogen Peroxide In Redox Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Hydrogen Peroxide In Redox Titration hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. Potassium permanganate acts as an oxidizing agent, oxidizing. what you need to know. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. To determine the concentration of hydrogen peroxide in. Hydrogen Peroxide In Redox Titration.
From hhsapchemistrybrueckner201112.blogspot.com
Heritage High School Mr. Brueckner's AP Chemistry Class 201112 Hydrogen Peroxide In Redox Titration Potassium permanganate acts as an oxidizing agent, oxidizing. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination. Hydrogen Peroxide In Redox Titration.
From studylib.net
The Potentiometric Titration of Hydrogen Peroxide Hydrogen Peroxide In Redox Titration Potassium permanganate acts as an oxidizing agent, oxidizing. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. what you need to know. redox titration is. Hydrogen Peroxide In Redox Titration.
From springgrovegarden.blogspot.com
Determining The Concentration Of Hydrogen Peroxide By Redox Titration Hydrogen Peroxide In Redox Titration what you need to know. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. hydrogen peroxide in a diluted portion of. Hydrogen Peroxide In Redox Titration.
From www.studocu.com
Analysis of hydrogen peroxide a redox titration Analysis of Hydrogen Hydrogen Peroxide In Redox Titration we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. redox titration is a laboratory method of determining the concentration of a given analyte by causing a. Hydrogen Peroxide In Redox Titration.
From www.chegg.com
Solved Lab 7. Redox Titration of Hydrogen peroxide Hydrogen Peroxide In Redox Titration hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. what you need to know. To determine the concentration of hydrogen peroxide in solution, the method called. Hydrogen Peroxide In Redox Titration.
From www.scribd.com
Hydrogen Peroxide Determination by Redox Titration Hydrogen Peroxide In Redox Titration what you need to know. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an. Hydrogen Peroxide In Redox Titration.
From stepmomdialogues.blogspot.com
Determining The Concentration Of Hydrogen Peroxide By Redox Titration Hydrogen Peroxide In Redox Titration hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. what you need to know. hydrogen peroxide. Hydrogen Peroxide In Redox Titration.
From www.chegg.com
Solved Titration of Hydrogen Peroxide Obiective In this Hydrogen Peroxide In Redox Titration hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. redox titration is a laboratory method of determining the concentration of a given analyte by causing a redox reaction between the. Potassium permanganate acts as. Hydrogen Peroxide In Redox Titration.
From www.flinnsci.com
Analysis of Hydrogen Peroxide A Redox Titration—ChemTopic™ Lab Hydrogen Peroxide In Redox Titration To determine the concentration of hydrogen peroxide in solution, the method called manganometry. hydrogen peroxide (h 2 o 2) is titrated with potassium permanganate in an acidic medium. what you need to know. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. . Hydrogen Peroxide In Redox Titration.
From www.coursehero.com
[Solved] . Redox titrations are used to determine the amounts of Hydrogen Peroxide In Redox Titration hydrogen peroxide in a diluted portion of the sample is quantitatively oxidized by titration with a potassium. we present the determination of hydrogen peroxide decomposition rate by temperature treatment at 96 °c for 16 hours, the determination of ph and. To determine the concentration of hydrogen peroxide in solution, the method called manganometry. Potassium permanganate acts as an. Hydrogen Peroxide In Redox Titration.