Increase Pressure Decrease Boiling Point . the dependence of the boiling point on the external pressure can often be very useful. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. As elevation increases, atmospheric pressure decreases. therefore, increasing the pressure displaces the equilibrium (c.f. Le chatelier) towards liquid, and causes condensation. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. In an open system this is called atmospheric pressure. when the vapour pressure equals atmospheric pressure, the liquid boils. the pressure of gas above a liquid affects the boiling point. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from.
from www.numerade.com
when the vapour pressure equals atmospheric pressure, the liquid boils. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. therefore, increasing the pressure displaces the equilibrium (c.f. As elevation increases, atmospheric pressure decreases. the pressure of gas above a liquid affects the boiling point. Le chatelier) towards liquid, and causes condensation. for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from.
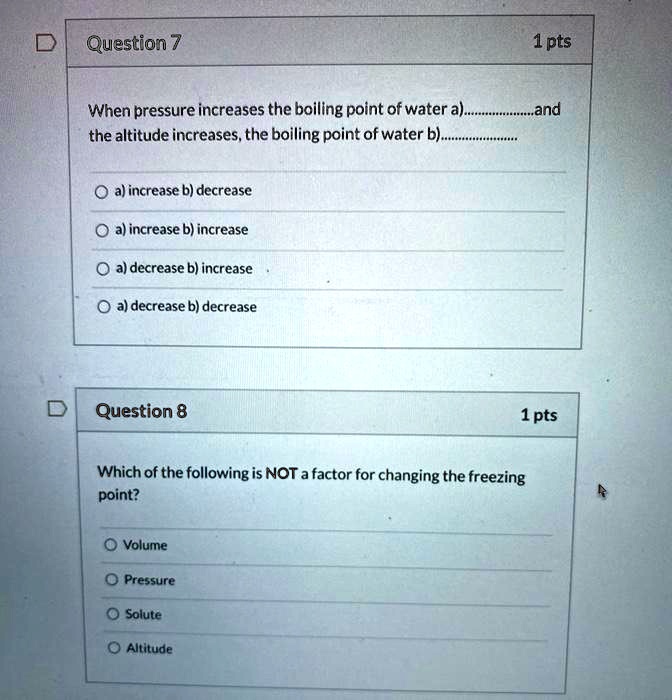
SOLVED Question 7 1pts When pressure increases the boiling point = of
Increase Pressure Decrease Boiling Point for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. when the vapour pressure equals atmospheric pressure, the liquid boils. therefore, increasing the pressure displaces the equilibrium (c.f. the pressure of gas above a liquid affects the boiling point. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. In an open system this is called atmospheric pressure. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. the dependence of the boiling point on the external pressure can often be very useful. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Le chatelier) towards liquid, and causes condensation. As elevation increases, atmospheric pressure decreases. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out.
From chem.libretexts.org
13.8 FreezingPoint Depression and BoilingPoint Elevation of Increase Pressure Decrease Boiling Point As elevation increases, atmospheric pressure decreases. therefore, increasing the pressure displaces the equilibrium (c.f. the pressure of gas above a liquid affects the boiling point. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. when you reduce the pressure of its surroundings, it takes less vapor pressure. Increase Pressure Decrease Boiling Point.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills Increase Pressure Decrease Boiling Point the dependence of the boiling point on the external pressure can often be very useful. therefore, increasing the pressure displaces the equilibrium (c.f. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at. Increase Pressure Decrease Boiling Point.
From exomkbouq.blob.core.windows.net
What Is Boiling Point Elevation Constant at Ronnie Moore blog Increase Pressure Decrease Boiling Point increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. Le chatelier) towards liquid, and causes condensation. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. the pressure of gas above a liquid affects the boiling point.. Increase Pressure Decrease Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Increase Pressure Decrease Boiling Point when the vapour pressure equals atmospheric pressure, the liquid boils. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Le chatelier) towards liquid, and causes condensation. therefore, increasing the pressure displaces the equilibrium (c.f. In an open system this is called atmospheric pressure. the pressure of gas above a liquid. Increase Pressure Decrease Boiling Point.
From www.youtube.com
Boiling point and external pressure YouTube Increase Pressure Decrease Boiling Point increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. Le chatelier) towards liquid, and causes condensation. In an open system this is called atmospheric pressure. the pressure of gas above a liquid affects the boiling point. therefore, increasing the pressure displaces the equilibrium (c.f. for example,. Increase Pressure Decrease Boiling Point.
From www.slideserve.com
PPT Fundamentals of Heat Transfer PowerPoint Presentation, free Increase Pressure Decrease Boiling Point for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. the pressure of gas above a liquid affects the boiling point. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Some liquids have such high normal boiling. Increase Pressure Decrease Boiling Point.
From slideplayer.com
WATER And Solution Formation ppt download Increase Pressure Decrease Boiling Point As elevation increases, atmospheric pressure decreases. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. Le chatelier) towards liquid, and causes condensation. the dependence of the boiling point on the external pressure can often be very useful. Some liquids have such high normal boiling points that they begin to decompose before distillation. Increase Pressure Decrease Boiling Point.
From dxofxnylq.blob.core.windows.net
What Happens To The Boiling Point Of Liquid When Atmospheric Pressure Increase Pressure Decrease Boiling Point Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the.. Increase Pressure Decrease Boiling Point.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Increase Pressure Decrease Boiling Point Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. the pressure of gas above a liquid affects the boiling point. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. when you reduce the pressure of its surroundings, it takes less. Increase Pressure Decrease Boiling Point.
From pakmcqs.com
With the increase of pressure, the boiling point of any substance Increase Pressure Decrease Boiling Point As elevation increases, atmospheric pressure decreases. In an open system this is called atmospheric pressure. for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. when the vapour. Increase Pressure Decrease Boiling Point.
From mavink.com
Water Boiling Point Pressure Chart Increase Pressure Decrease Boiling Point In an open system this is called atmospheric pressure. therefore, increasing the pressure displaces the equilibrium (c.f. the dependence of the boiling point on the external pressure can often be very useful. for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. . Increase Pressure Decrease Boiling Point.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Increase Pressure Decrease Boiling Point the pressure of gas above a liquid affects the boiling point. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. In an open system this is called atmospheric pressure. therefore, increasing the pressure displaces the equilibrium (c.f. when the vapour pressure equals atmospheric pressure, the liquid boils. for example,. Increase Pressure Decrease Boiling Point.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Increase Pressure Decrease Boiling Point the pressure of gas above a liquid affects the boiling point. In an open system this is called atmospheric pressure. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. Le chatelier) towards liquid, and causes condensation. when the vapour pressure equals atmospheric pressure, the liquid boils.. Increase Pressure Decrease Boiling Point.
From facts.net
20 Fascinating Facts About Boiling Point Elevation Increase Pressure Decrease Boiling Point In an open system this is called atmospheric pressure. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. As elevation increases, atmospheric pressure decreases. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. for example, an increase in pressure from 100 to. Increase Pressure Decrease Boiling Point.
From www.slideserve.com
PPT Intermolecular forces Generalizing properties PowerPoint Increase Pressure Decrease Boiling Point the dependence of the boiling point on the external pressure can often be very useful. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. Le chatelier) towards liquid, and causes condensation. As elevation increases, atmospheric pressure decreases. In an open system this is called atmospheric pressure. therefore,. Increase Pressure Decrease Boiling Point.
From exomkbouq.blob.core.windows.net
What Is Boiling Point Elevation Constant at Ronnie Moore blog Increase Pressure Decrease Boiling Point increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. the dependence of the boiling point on the external pressure can often be very useful. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. Le chatelier) towards liquid, and. Increase Pressure Decrease Boiling Point.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube Increase Pressure Decrease Boiling Point therefore, increasing the pressure displaces the equilibrium (c.f. for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. Some liquids have such high. Increase Pressure Decrease Boiling Point.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Increase Pressure Decrease Boiling Point the dependence of the boiling point on the external pressure can often be very useful. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. therefore, increasing the pressure displaces the equilibrium (c.f. As elevation increases, atmospheric pressure decreases. Some liquids have such high normal boiling points. Increase Pressure Decrease Boiling Point.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point Increase Pressure Decrease Boiling Point therefore, increasing the pressure displaces the equilibrium (c.f. for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. Chemists often purify liquids by boiling. Increase Pressure Decrease Boiling Point.
From sciencenotes.org
Boyle's Law Definition, Formula, Example Increase Pressure Decrease Boiling Point when the vapour pressure equals atmospheric pressure, the liquid boils. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. Le chatelier) towards liquid, and causes condensation. In an open system this is called atmospheric pressure. when you reduce the pressure of its surroundings, it takes less vapor pressure. Increase Pressure Decrease Boiling Point.
From diagramlibpirriconeu1m.z13.web.core.windows.net
Initial Boiling Point And Final Boiling Point Increase Pressure Decrease Boiling Point when the vapour pressure equals atmospheric pressure, the liquid boils. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. the dependence of the boiling point on the external pressure can often be very useful. for example, an increase in pressure from 100 to 200 mmhg results in. Increase Pressure Decrease Boiling Point.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download Increase Pressure Decrease Boiling Point the pressure of gas above a liquid affects the boiling point. Le chatelier) towards liquid, and causes condensation. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point. Increase Pressure Decrease Boiling Point.
From brainly.in
Why boiling point decreses then vapor pressure increases? Brainly.in Increase Pressure Decrease Boiling Point for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. As elevation increases, atmospheric pressure decreases. In an open system this is called atmospheric pressure.. Increase Pressure Decrease Boiling Point.
From www.numerade.com
SOLVED Question 7 1pts When pressure increases the boiling point = of Increase Pressure Decrease Boiling Point for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. In an open system this is called atmospheric pressure. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. Chemists often purify liquids by boiling them. Increase Pressure Decrease Boiling Point.
From slideplayer.com
Changes of State Chapter 10 Section ppt download Increase Pressure Decrease Boiling Point Le chatelier) towards liquid, and causes condensation. As elevation increases, atmospheric pressure decreases. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. when the vapour pressure equals atmospheric pressure, the liquid boils. for example, an increase in pressure from 100 to 200 mmhg results in an increase in. Increase Pressure Decrease Boiling Point.
From www.slideserve.com
PPT Energy, Temperature, Phase Changes PowerPoint Increase Pressure Decrease Boiling Point therefore, increasing the pressure displaces the equilibrium (c.f. In an open system this is called atmospheric pressure. when the vapour pressure equals atmospheric pressure, the liquid boils. Le chatelier) towards liquid, and causes condensation. the pressure of gas above a liquid affects the boiling point. the dependence of the boiling point on the external pressure can. Increase Pressure Decrease Boiling Point.
From www.chegg.com
Solved 8. What happens to each of the four when a solute is Increase Pressure Decrease Boiling Point when the vapour pressure equals atmospheric pressure, the liquid boils. the dependence of the boiling point on the external pressure can often be very useful. As elevation increases, atmospheric pressure decreases. the pressure of gas above a liquid affects the boiling point. for example, an increase in pressure from 100 to 200 mmhg results in an. Increase Pressure Decrease Boiling Point.
From kunduz.com
[ANSWERED] ier in pressure cooke 200 a Boiling point increases with Increase Pressure Decrease Boiling Point As elevation increases, atmospheric pressure decreases. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. therefore, increasing the pressure displaces the equilibrium (c.f. the dependence of the boiling point on the external pressure can often be very useful. for example, an increase in pressure from 100. Increase Pressure Decrease Boiling Point.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Increase Pressure Decrease Boiling Point increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. therefore, increasing the pressure displaces the equilibrium (c.f. when the vapour pressure equals atmospheric pressure, the liquid boils. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. As. Increase Pressure Decrease Boiling Point.
From www.numerade.com
SOLVEDIncrease of pressure (a) always increases the boiling point of Increase Pressure Decrease Boiling Point for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. Chemists often purify liquids by boiling them and collecting the vapor, a process known as. Increase Pressure Decrease Boiling Point.
From www.slideserve.com
PPT PRINCIPLES OF HEAT TRANSFER PowerPoint Presentation, free Increase Pressure Decrease Boiling Point Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. the dependence of the boiling point on the external pressure can often be very useful. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. the pressure of gas above a. Increase Pressure Decrease Boiling Point.
From brainly.com
Air pressure decreases as elevation increases. Describe how the boiling Increase Pressure Decrease Boiling Point Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. when the vapour pressure equals atmospheric pressure, the liquid boils. Some liquids have such high normal boiling points that they begin to decompose before distillation can be carried out. In an open system this is called atmospheric pressure. Le chatelier) towards liquid, and. Increase Pressure Decrease Boiling Point.
From www.youtube.com
BoilingPoint Increase and FreezingPoint Decrease YouTube Increase Pressure Decrease Boiling Point therefore, increasing the pressure displaces the equilibrium (c.f. when you reduce the pressure of its surroundings, it takes less vapor pressure to equal that, which occurs at a. when the vapour pressure equals atmospheric pressure, the liquid boils. Le chatelier) towards liquid, and causes condensation. the dependence of the boiling point on the external pressure can. Increase Pressure Decrease Boiling Point.
From brainly.in
As pressure increase boiling point of liquid increase or decrease Increase Pressure Decrease Boiling Point increasing the pressure has the overall effect of reducing the enthalpy of vaporization, until it becomes zero at the. Chemists often purify liquids by boiling them and collecting the vapor, a process known as distillation. the pressure of gas above a liquid affects the boiling point. when you reduce the pressure of its surroundings, it takes less. Increase Pressure Decrease Boiling Point.
From www.slideshare.net
Air pressure Increase Pressure Decrease Boiling Point therefore, increasing the pressure displaces the equilibrium (c.f. for example, an increase in pressure from 100 to 200 mmhg results in an increase in the boiling point of water of 15°c (from. the pressure of gas above a liquid affects the boiling point. increasing the pressure has the overall effect of reducing the enthalpy of vaporization,. Increase Pressure Decrease Boiling Point.