Why Does Thermite Reaction Work . a much more favorable enthalpy provokes and carries the reaction. Iron(iii) oxide + aluminium → aluminium oxide + iron. Here's how you can perform the thermite. thermite heaters are used when working with our bismuth alloys for the following reasons: the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. The resulting heat from the demonstration itself melts the. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. This shows that aluminium is above iron in the reactivity series. A large amount of energy is. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. the thermite reaction is a highly exothermic reaction in which metal essentially burns.
from rafeechemistry.weebly.com
the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. This shows that aluminium is above iron in the reactivity series. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. Here's how you can perform the thermite. a much more favorable enthalpy provokes and carries the reaction. the thermite reaction is a highly exothermic reaction in which metal essentially burns. The resulting heat from the demonstration itself melts the. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). thermite heaters are used when working with our bismuth alloys for the following reasons:
History and Uses Chemistry AssignmentThermite Reaction
Why Does Thermite Reaction Work Here's how you can perform the thermite. A large amount of energy is. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. This shows that aluminium is above iron in the reactivity series. The resulting heat from the demonstration itself melts the. the thermite reaction is a highly exothermic reaction in which metal essentially burns. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. a much more favorable enthalpy provokes and carries the reaction. Iron(iii) oxide + aluminium → aluminium oxide + iron. Here's how you can perform the thermite. thermite heaters are used when working with our bismuth alloys for the following reasons:
From sciencenotes.org
Thermite Reaction Chemistry Demonstration Why Does Thermite Reaction Work thermite heaters are used when working with our bismuth alloys for the following reasons: A large amount of energy is. The resulting heat from the demonstration itself melts the. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). This shows that aluminium. Why Does Thermite Reaction Work.
From www.youtube.com
Thermite reaction YouTube Why Does Thermite Reaction Work As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. a much more favorable enthalpy provokes and carries the reaction. Here's how you can perform the thermite. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). This shows. Why Does Thermite Reaction Work.
From www.slideshare.net
Thermite reactions Why Does Thermite Reaction Work The resulting heat from the demonstration itself melts the. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. thermite heaters are used when working. Why Does Thermite Reaction Work.
From studylib.net
Thermite Reaction And Activation Energy Why Does Thermite Reaction Work a much more favorable enthalpy provokes and carries the reaction. the thermite reaction is a highly exothermic reaction in which metal essentially burns. The resulting heat from the demonstration itself melts the. the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. A large amount of energy is. Iron(iii) oxide + aluminium. Why Does Thermite Reaction Work.
From exothermicredoxreaction.weebly.com
Balancing Thermite Reaction Why Does Thermite Reaction Work Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Here's how you can perform the thermite. A large amount of energy is. The resulting heat from the demonstration itself melts the. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and. Why Does Thermite Reaction Work.
From www.youtube.com
The Thermite Reaction YouTube Why Does Thermite Reaction Work A large amount of energy is. Here's how you can perform the thermite. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. a much more favorable enthalpy provokes and carries the reaction. The resulting heat from the demonstration itself melts the. the thermite reaction is a spectacular exothermic chemical. Why Does Thermite Reaction Work.
From www.slideserve.com
PPT Thermite reaction PowerPoint Presentation, free download ID6672045 Why Does Thermite Reaction Work As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. a much more favorable enthalpy provokes and carries the reaction. This shows that aluminium is above iron in the reactivity series. A large amount of energy is. thermite heaters are used when working with our bismuth alloys for the following reasons: the thermite. Why Does Thermite Reaction Work.
From www.youtube.com
Examining The Thermite Reaction Front YouTube Why Does Thermite Reaction Work The resulting heat from the demonstration itself melts the. Iron(iii) oxide + aluminium → aluminium oxide + iron. This shows that aluminium is above iron in the reactivity series. a much more favorable enthalpy provokes and carries the reaction. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. thermite heaters are used when. Why Does Thermite Reaction Work.
From www.cruxweld.com
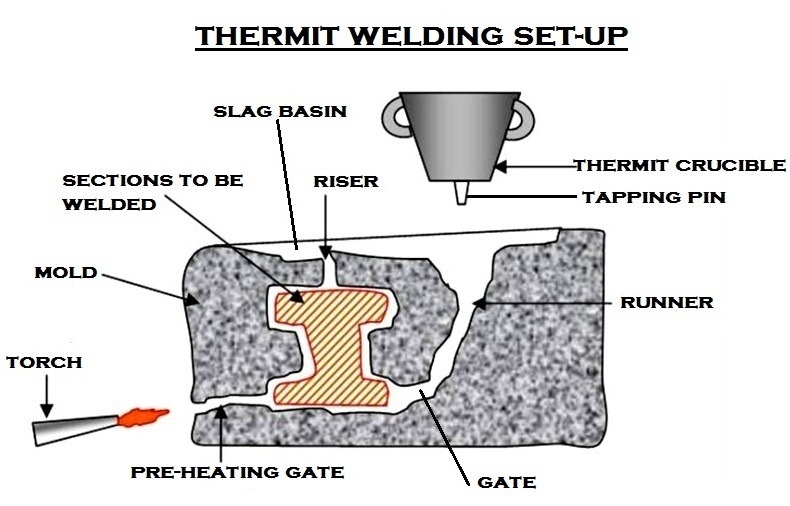
Thermit Welding Operation and Steps [With Setup Diagram] cruxweld Why Does Thermite Reaction Work the thermite reaction is a highly exothermic reaction in which metal essentially burns. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. A large amount of energy is. This shows that aluminium is above iron in the reactivity series. thermite heaters are used when working with our bismuth alloys for the following reasons:. Why Does Thermite Reaction Work.
From www.thoughtco.com
Thermite Reaction Instructions and Chemistry Why Does Thermite Reaction Work A large amount of energy is. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. The resulting heat from the demonstration itself melts the. a much more favorable enthalpy provokes and carries the reaction. the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. the thermite reaction. Why Does Thermite Reaction Work.
From www.youtube.com
THERMITE REACTION PROCESS FOR REPAIRING RAILWAY TRACKS YouTube Why Does Thermite Reaction Work the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. This shows that aluminium is above iron in the reactivity series. Here's how you can perform the thermite. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. the thermite reaction is a highly exothermic reaction in which metal. Why Does Thermite Reaction Work.
From www.youtube.com
Thermite Reactions Compilation YouTube Why Does Thermite Reaction Work As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. Here's how you can perform the thermite. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting. Why Does Thermite Reaction Work.
From www.alamy.com
Thermite Reaction Stock Photo 14876653 Alamy Why Does Thermite Reaction Work thermite heaters are used when working with our bismuth alloys for the following reasons: A large amount of energy is. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). the thermite reaction is a highly exothermic reaction in which metal essentially. Why Does Thermite Reaction Work.
From kylesgarage.com
How does thermite welding work? Kyle's Garage Why Does Thermite Reaction Work Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Iron(iii) oxide + aluminium → aluminium oxide + iron. thermite heaters are used when working with our bismuth alloys for the following reasons: the thermite reaction is initiated by the heat released. Why Does Thermite Reaction Work.
From www.chemistrylearner.com
Thermite Reaction Definition, Formula, and Applications Why Does Thermite Reaction Work the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. thermite heaters are used when working with our bismuth alloys for the following reasons: Here's how you can perform the thermite. This shows that aluminium. Why Does Thermite Reaction Work.
From www.youtube.com
Thermite reaction in slowmo YouTube Why Does Thermite Reaction Work This shows that aluminium is above iron in the reactivity series. thermite heaters are used when working with our bismuth alloys for the following reasons: A large amount of energy is. Iron(iii) oxide + aluminium → aluminium oxide + iron. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. Once underway, the reaction is. Why Does Thermite Reaction Work.
From www.slideshare.net
Thermite reactions Why Does Thermite Reaction Work the thermite reaction is a highly exothermic reaction in which metal essentially burns. a much more favorable enthalpy provokes and carries the reaction. Here's how you can perform the thermite. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. Once underway, the reaction is highly exothermic, rapidly reaching temperatures. Why Does Thermite Reaction Work.
From www.thoughtco.com
Thermite Reaction Instructions and Chemistry Why Does Thermite Reaction Work thermite heaters are used when working with our bismuth alloys for the following reasons: The resulting heat from the demonstration itself melts the. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. This shows that aluminium is above iron in the reactivity series. the thermite reaction is initiated by the heat released from. Why Does Thermite Reaction Work.
From rafeechemistry.weebly.com
History and Uses Chemistry AssignmentThermite Reaction Why Does Thermite Reaction Work As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. Here's how you can perform the thermite. the thermite reaction is a highly exothermic reaction in which metal essentially burns. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. Once underway, the reaction is highly exothermic,. Why Does Thermite Reaction Work.
From www.youtube.com
Thermite Reaction YouTube Why Does Thermite Reaction Work The resulting heat from the demonstration itself melts the. the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. Iron(iii) oxide + aluminium → aluminium oxide + iron. thermite heaters are used when working with our bismuth alloys for the following reasons: A large amount of energy is. Once underway, the reaction is. Why Does Thermite Reaction Work.
From www.youtube.com
Science. Ep 1 S1 "Thermite Reaction" YouTube Why Does Thermite Reaction Work the thermite reaction is a highly exothermic reaction in which metal essentially burns. Here's how you can perform the thermite. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting. Why Does Thermite Reaction Work.
From mavink.com
Thermite Reaction Diagram Why Does Thermite Reaction Work A large amount of energy is. thermite heaters are used when working with our bismuth alloys for the following reasons: This shows that aluminium is above iron in the reactivity series. the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. The resulting heat from the demonstration itself melts the. the thermite. Why Does Thermite Reaction Work.
From www.youtube.com
thermit welding thermite welding process Thermite welding reaction Why Does Thermite Reaction Work Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. a much more favorable enthalpy provokes and carries the reaction. the thermite reaction is a spectacular exothermic chemical. Why Does Thermite Reaction Work.
From www.youtube.com
THERMITE REACTION (thermite reaction definition) YouTube Why Does Thermite Reaction Work the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. a much more favorable enthalpy provokes and carries the reaction. The resulting heat from the demonstration itself melts the. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. This shows that aluminium is above iron in the reactivity. Why Does Thermite Reaction Work.
From weldingheadquarters.com
How Does Thermite Welding Work? Welding Headquarters Why Does Thermite Reaction Work the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). Iron(iii) oxide + aluminium → aluminium oxide + iron. a much more favorable enthalpy provokes. Why Does Thermite Reaction Work.
From www.youtube.com
Thermite Reaction AP Chemistry Demo 2012 YouTube Why Does Thermite Reaction Work Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. a much more favorable enthalpy provokes and carries the reaction. the thermite reaction is. Why Does Thermite Reaction Work.
From www.slideserve.com
PPT Thermite reaction PowerPoint Presentation, free download ID6672045 Why Does Thermite Reaction Work the thermite reaction is a highly exothermic reaction in which metal essentially burns. the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. Here's how you can perform the thermite. The resulting heat from the demonstration itself melts the. This shows that aluminium is above iron in the reactivity series. a much. Why Does Thermite Reaction Work.
From www.slideshare.net
Thermite reactions Why Does Thermite Reaction Work Iron(iii) oxide + aluminium → aluminium oxide + iron. A large amount of energy is. Here's how you can perform the thermite. This shows that aluminium is above iron in the reactivity series. a much more favorable enthalpy provokes and carries the reaction. thermite heaters are used when working with our bismuth alloys for the following reasons: . Why Does Thermite Reaction Work.
From www.bartleby.com
Answered The thermite reaction occurs when… bartleby Why Does Thermite Reaction Work Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). thermite heaters are used when working with our bismuth alloys for the following reasons: a much more favorable enthalpy provokes and carries the reaction. The resulting heat from the demonstration itself melts. Why Does Thermite Reaction Work.
From www.popularmechanics.com
Slow Motion Thermite Reaction Video How Does Thermite Work? Why Does Thermite Reaction Work A large amount of energy is. the thermite reaction is initiated by the heat released from the mixture of potassium permanganate and glycerine. Here's how you can perform the thermite. This shows that aluminium is above iron in the reactivity series. The resulting heat from the demonstration itself melts the. a much more favorable enthalpy provokes and carries. Why Does Thermite Reaction Work.
From blog.thepipingmart.com
What is Thermite Welding, and Why Is Aluminium Used? Why Does Thermite Reaction Work A large amount of energy is. Iron(iii) oxide + aluminium → aluminium oxide + iron. the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. a much more favorable enthalpy provokes and carries the reaction. thermite heaters are used when working with our bismuth alloys for the following reasons: Here's how you. Why Does Thermite Reaction Work.
From www.youtube.com
The thermite reaction YouTube Why Does Thermite Reaction Work the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. thermite heaters are used when working with our bismuth alloys for the following reasons: The resulting heat from the demonstration itself melts the. This shows that aluminium is above iron in the reactivity series. Iron(iii) oxide + aluminium → aluminium oxide + iron.. Why Does Thermite Reaction Work.
From www.thoughtco.com
Thermite Reaction Instructions and Chemistry Why Does Thermite Reaction Work Here's how you can perform the thermite. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting point of iron (1535 °c). This shows that aluminium is above iron in the reactivity series. a much more favorable enthalpy provokes and carries the reaction. the thermite reaction is. Why Does Thermite Reaction Work.
From www.thoughtco.com
Thermite Reaction Instructions and Chemistry Why Does Thermite Reaction Work the thermite reaction is a highly exothermic reaction in which metal essentially burns. The resulting heat from the demonstration itself melts the. the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. the thermite reaction is initiated by. Why Does Thermite Reaction Work.
From www.slideserve.com
PPT The Thermite Process PowerPoint Presentation, free download ID Why Does Thermite Reaction Work the thermite reaction is a spectacular exothermic chemical reaction that makes an exciting chemistry demonstration. The resulting heat from the demonstration itself melts the. As a demonstration, the reaction illustrates the metal reactivity series, oxidation, and exothermic reaction. Once underway, the reaction is highly exothermic, rapidly reaching temperatures as high as 2000 °c, well in excess of the melting. Why Does Thermite Reaction Work.