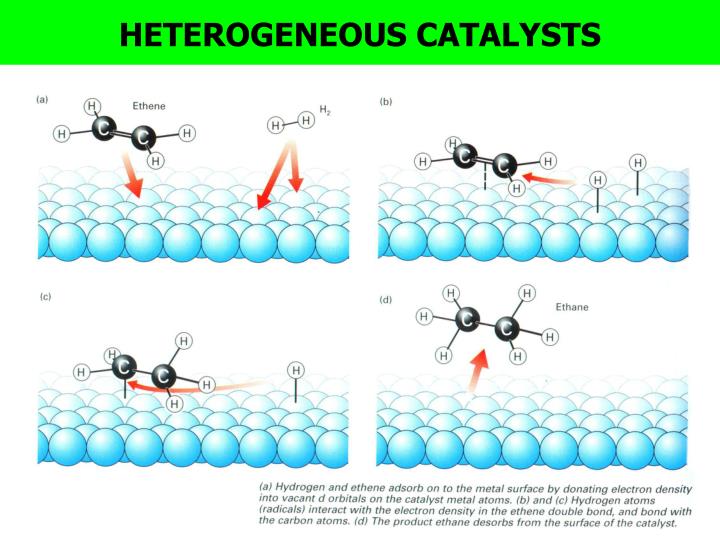
Chemistry Catalysis Homogeneous . It interacts with a reactant to form an intermediate substance, which then. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). In homogeneous catalysis, a catalyst is in the same phase as the reactants. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. A homogeneous catalyst is present in the same phase as the reactants. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. A homogeneous catalyst is present in the same phase as the reactants. As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. Homogeneous catalysis and heterogeneous catalysis. Reactions that are facilitated by catalysts can be divided into two major classes:
from www.slideserve.com
Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. It interacts with a reactant to form an intermediate substance, which then. A homogeneous catalyst is present in the same phase as the reactants. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). Homogeneous catalysis and heterogeneous catalysis. Reactions that are facilitated by catalysts can be divided into two major classes: In homogeneous catalysis, a catalyst is in the same phase as the reactants.
PPT Starter 1)Definition of catalysts 2) Difference between
Chemistry Catalysis Homogeneous Reactions that are facilitated by catalysts can be divided into two major classes: A homogeneous catalyst is present in the same phase as the reactants. It interacts with a reactant to form an intermediate substance, which then. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. A homogeneous catalyst is present in the same phase as the reactants. As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. Homogeneous catalysis and heterogeneous catalysis. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. In homogeneous catalysis, a catalyst is in the same phase as the reactants. Reactions that are facilitated by catalysts can be divided into two major classes:
From www.slideserve.com
PPT Catalysis for Chemical Engineers PowerPoint Presentation, free Chemistry Catalysis Homogeneous Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. It interacts with a reactant to form an intermediate substance, which then. A homogeneous catalyst is present in the same phase as the reactants. A homogeneous catalyst is present in the same phase as the reactants. The number of. Chemistry Catalysis Homogeneous.
From www.youtube.com
Chemical Made Easy Homogeneous and Heterogeneous Catalysts Chemistry Catalysis Homogeneous A homogeneous catalyst is present in the same phase as the reactants. In homogeneous catalysis, a catalyst is in the same phase as the reactants. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. A homogeneous catalyst is present in the same phase as the reactants. The number. Chemistry Catalysis Homogeneous.
From meaningkosh.com
Catalytic Meaning In Hindi MeaningKosh Chemistry Catalysis Homogeneous Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). Homogeneous catalysis, by. Chemistry Catalysis Homogeneous.
From www.slideshare.net
Homogeneous catalysis [ MPHARM, MSC, BPHARM, BSC] Chemistry Catalysis Homogeneous Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. Homogeneous catalysis and heterogeneous catalysis. A homogeneous catalyst is present in the same phase as the reactants. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. In homogeneous catalysis, a catalyst is in. Chemistry Catalysis Homogeneous.
From artenaescola.org.br
Spravedlnost pomáhat Jahoda contribution of catalysed reactions to Chemistry Catalysis Homogeneous In homogeneous catalysis, a catalyst is in the same phase as the reactants. Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. Reactions that are. Chemistry Catalysis Homogeneous.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Chemistry Catalysis Homogeneous In homogeneous catalysis, a catalyst is in the same phase as the reactants. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. It interacts with a reactant to form an intermediate substance, which then.. Chemistry Catalysis Homogeneous.
From www.ucl.ac.uk
Catalysis and Reaction Engineering UCL Department of Chemical Chemistry Catalysis Homogeneous As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). A homogeneous catalyst is present in the same phase as the reactants. The number of collisions between reactants and catalyst is at a maximum because the catalyst is. Chemistry Catalysis Homogeneous.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID3996834 Chemistry Catalysis Homogeneous Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. In homogeneous catalysis, a catalyst is in the same phase as the reactants. As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. Homogeneous catalysis and heterogeneous catalysis. Said “industrial. Chemistry Catalysis Homogeneous.
From www.slideserve.com
PPT Homogeneous catalysis PowerPoint Presentation, free download ID Chemistry Catalysis Homogeneous Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly. Chemistry Catalysis Homogeneous.
From www.linstitute.net
IB DP Chemistry HL复习笔记6.1.8 Energy Profiles & Catalysis翰林国际教育 Chemistry Catalysis Homogeneous Homogeneous catalysis and heterogeneous catalysis. As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have.. Chemistry Catalysis Homogeneous.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Chemistry Catalysis Homogeneous The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. A homogeneous catalyst is present in the same phase as the reactants. In homogeneous catalysis, a catalyst is in the same phase as the reactants. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates. Chemistry Catalysis Homogeneous.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Chemistry Catalysis Homogeneous It interacts with a reactant to form an intermediate substance, which then. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). Reactions that are facilitated by catalysts can be divided into two major classes: Homogeneous catalysis and heterogeneous catalysis. Homogeneous catalysis, by. Chemistry Catalysis Homogeneous.
From www.vrogue.co
Process Flowsheet For Biodiesel Production Using Homo vrogue.co Chemistry Catalysis Homogeneous Homogeneous catalysis and heterogeneous catalysis. Reactions that are facilitated by catalysts can be divided into two major classes: The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in. Chemistry Catalysis Homogeneous.
From jacyou.com
CO2変換のための均一触媒プロトタイプに基づく単原子触媒 Chemistry Catalysis Homogeneous In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). It interacts with a reactant to form an intermediate substance, which then. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. A homogeneous catalyst is present in the same phase as the reactants. Said “industrial applications of homogeneous catalysis are proven, and. Chemistry Catalysis Homogeneous.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Chemistry Catalysis Homogeneous A homogeneous catalyst is present in the same phase as the reactants. Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). Homogeneous catalysis and heterogeneous catalysis. Homogeneous catalysis, by definition, refers to a catalytic. Chemistry Catalysis Homogeneous.
From www.youtube.com
Types Of Homogeneous Catalysis YouTube Chemistry Catalysis Homogeneous Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. It interacts with a reactant to form an intermediate substance, which then. In homogeneous catalysis, a catalyst is in the same phase as the reactants. Homogeneous catalysis and heterogeneous catalysis. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). Reactions that are. Chemistry Catalysis Homogeneous.
From www.researchgate.net
Homogeneous vs heterogeneous catalysts. Design of heterogeneous Chemistry Catalysis Homogeneous Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. Homogeneous catalysis and heterogeneous catalysis. It interacts with a reactant to form an intermediate substance, which then. In homogeneous catalysis, a catalyst is in the same phase as the reactants. A homogeneous catalyst is present in the same phase as the reactants. A homogeneous catalyst is. Chemistry Catalysis Homogeneous.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Chemistry Catalysis Homogeneous In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). In homogeneous catalysis, a catalyst is in the same phase as the reactants. A homogeneous catalyst is present in the same phase as the reactants. Homogeneous catalysis and heterogeneous catalysis. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. Homogeneous catalysis, by. Chemistry Catalysis Homogeneous.
From ar.inspiredpencil.com
Catalyst Chemistry Catalysis Homogeneous Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. A homogeneous catalyst is present in the same phase as the reactants. Homogeneous catalysis and heterogeneous catalysis.. Chemistry Catalysis Homogeneous.
From www.cademix.org
Applications of Heterogeneous Catalysis in Industry Chemistry Catalysis Homogeneous It interacts with a reactant to form an intermediate substance, which then. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. Aqueous transition metal ions are a common example of homogeneous catalysts, as they. Chemistry Catalysis Homogeneous.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Chemistry Catalysis Homogeneous The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. In homogeneous catalysis, a catalyst is in the same phase as the reactants. A homogeneous catalyst is present in the same phase as the reactants. It interacts with a reactant to form an intermediate substance, which then. Homogeneous. Chemistry Catalysis Homogeneous.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Chemistry Catalysis Homogeneous Homogeneous catalysis and heterogeneous catalysis. In homogeneous catalysis, a catalyst is in the same phase as the reactants. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components. Chemistry Catalysis Homogeneous.
From chemistrydocs.com
Photocatalysis Types, Mechanism And Applications Chemistry Catalysis Homogeneous Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. Homogeneous catalysis and heterogeneous catalysis. It interacts with a reactant to form an intermediate substance, which then. As the name implies, homogeneous catalysts are. Chemistry Catalysis Homogeneous.
From blogs.rsc.org
Homogeneous catalyst made to act more like an enzyme Catalysis Chemistry Catalysis Homogeneous Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. A homogeneous catalyst is present in the same phase as the reactants. A homogeneous catalyst is present in the same phase as the reactants. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture.. Chemistry Catalysis Homogeneous.
From animalia-life.club
Mixtures Examples Chemistry Catalysis Homogeneous Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout. Chemistry Catalysis Homogeneous.
From www.differencebetween.com
Difference Between Homogeneous and Heterogeneous Reactions Compare Chemistry Catalysis Homogeneous In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. Said “industrial applications of. Chemistry Catalysis Homogeneous.
From www.slideshare.net
Catalysis Chemistry Catalysis Homogeneous Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. Homogeneous catalysis and heterogeneous catalysis. In homogeneous catalysis, a catalyst is in the same phase as the reactants. As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. It interacts with a reactant to form an intermediate. Chemistry Catalysis Homogeneous.
From www.youtube.com
What is a Homogeneous Catalyst? YouTube Chemistry Catalysis Homogeneous As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. It interacts with a reactant to form an intermediate substance, which then. A homogeneous catalyst is present in the same phase as the reactants. Reactions that are facilitated by catalysts can be divided into two major classes: The number of collisions. Chemistry Catalysis Homogeneous.
From dokumen.tips
(PDF) Organometallic Chemistry for Homogeneous Catalysis DOKUMEN.TIPS Chemistry Catalysis Homogeneous The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. A homogeneous catalyst. Chemistry Catalysis Homogeneous.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Chemistry Catalysis Homogeneous Homogeneous catalysis and heterogeneous catalysis. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a reaction and the catalyst components are. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). In homogeneous catalysis, a catalyst is in the same phase as the reactants. A homogeneous catalyst is present in the same. Chemistry Catalysis Homogeneous.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Chemistry Catalysis Homogeneous As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. A homogeneous catalyst is present in the same phase as the reactants. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. Homogeneous catalysis, by definition, refers to a catalytic system in which the substrates for a. Chemistry Catalysis Homogeneous.
From alchetron.com
Heterogeneous catalysis Alchetron, the free social encyclopedia Chemistry Catalysis Homogeneous Homogeneous catalysis and heterogeneous catalysis. As the name implies, homogeneous catalysts are present in the same phase (gas or liquid solution) as the reactants. Aqueous transition metal ions are a common example of homogeneous catalysts, as they have. In homogeneous catalysis, a catalyst is in the same phase as the reactants. Said “industrial applications of homogeneous catalysis are proven, and. Chemistry Catalysis Homogeneous.
From news.virginia.edu
University of Virginia Researchers Uncover New Catalysis Site UVA Today Chemistry Catalysis Homogeneous A homogeneous catalyst is present in the same phase as the reactants. Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. Reactions that are facilitated by catalysts can be divided into two major classes: In homogeneous catalysis, a catalyst is in the same phase as the reactants.. Chemistry Catalysis Homogeneous.
From www.essentialchemicalindustry.org
Catalysis in industry Chemistry Catalysis Homogeneous It interacts with a reactant to form an intermediate substance, which then. In homogeneous catalysis, a catalyst is in the same phase as the reactants. A homogeneous catalyst is present in the same phase as the reactants. Homogeneous catalysis and heterogeneous catalysis. Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.”. Chemistry Catalysis Homogeneous.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Chemistry Catalysis Homogeneous Said “industrial applications of homogeneous catalysis are proven, and a much wider application in the future is anticipated.” growth in the area. A homogeneous catalyst is present in the same phase as the reactants. A homogeneous catalyst is present in the same phase as the reactants. In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). Reactions. Chemistry Catalysis Homogeneous.