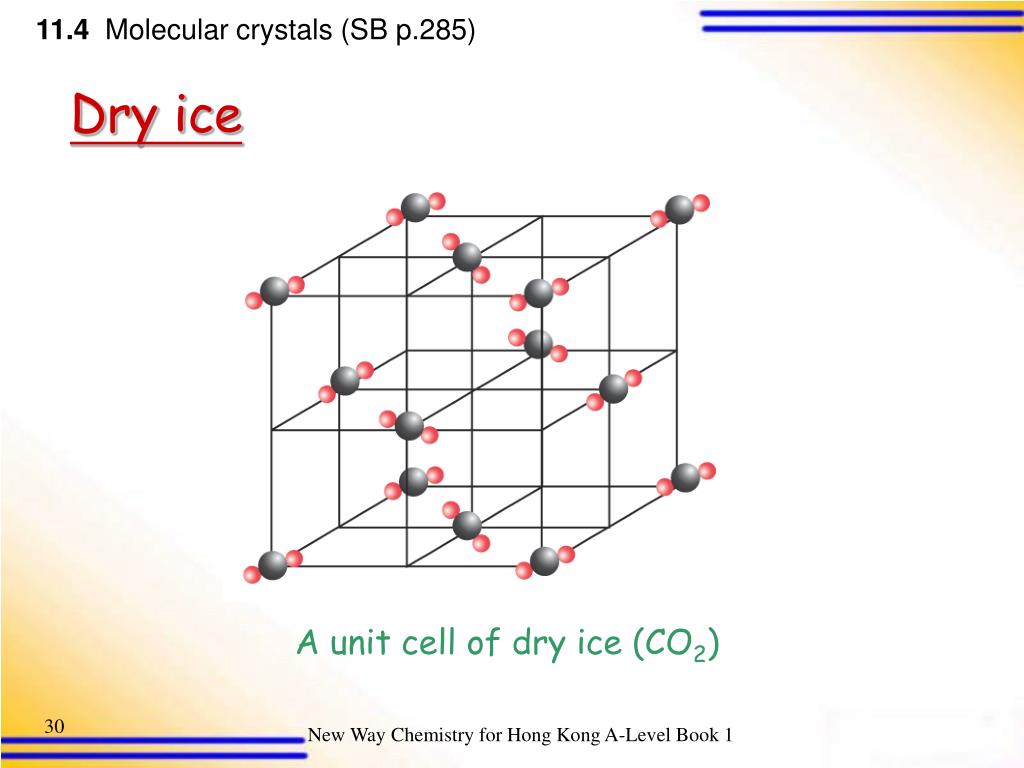
Crystal Structure Of Dry Ice . a classic study found that crystalline ice adopts an amorphous form when compressed. Plane wave cutoffs of 160 and 960 ry were used for. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. The equilibrium structure to which a material crystallizes under given conditions of temperature and. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. crystal structure of ice ih. Experiments now find that alternative phase. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig.
from www.slideserve.com
almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. crystal structure of ice ih. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. The equilibrium structure to which a material crystallizes under given conditions of temperature and. Experiments now find that alternative phase. crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. a classic study found that crystalline ice adopts an amorphous form when compressed. Plane wave cutoffs of 160 and 960 ry were used for.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
Crystal Structure Of Dry Ice a classic study found that crystalline ice adopts an amorphous form when compressed. crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. Plane wave cutoffs of 160 and 960 ry were used for. The equilibrium structure to which a material crystallizes under given conditions of temperature and. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. a classic study found that crystalline ice adopts an amorphous form when compressed. Experiments now find that alternative phase. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. crystal structure of ice ih.
From www.nature.com
A twist in the tale of the structure of ice Crystal Structure Of Dry Ice crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. crystal structure of ice ih. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. The equilibrium structure to which a material crystallizes under. Crystal Structure Of Dry Ice.
From www3.nd.edu
Structure of Ice Crystal Structure Of Dry Ice The equilibrium structure to which a material crystallizes under given conditions of temperature and. crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. Experiments now find that alternative phase. crystal structure. Crystal Structure Of Dry Ice.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Crystal Structure Of Dry Ice Experiments now find that alternative phase. crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. Plane wave cutoffs of 160 and 960 ry were used for. a classic study found that crystalline ice adopts an amorphous form when compressed. almost all ice is crystalline, and much natural ice is. Crystal Structure Of Dry Ice.
From www.pinterest.com
The Sixfold Nature of Snow Snow images, Crystals, Snow Crystal Structure Of Dry Ice The equilibrium structure to which a material crystallizes under given conditions of temperature and. Experiments now find that alternative phase. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. In the model we can clearly see that each o atom is surrounded. Crystal Structure Of Dry Ice.
From www.nisenet.org
Scientific Image Ice models NISE Network Crystal Structure Of Dry Ice a classic study found that crystalline ice adopts an amorphous form when compressed. crystal structure of ice ih. The equilibrium structure to which a material crystallizes under given conditions of temperature and. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. crystalline dry ice has a cubic. Crystal Structure Of Dry Ice.
From blogs.egu.eu
Cryospheric Sciences Image of the Week The Journey of a Snowflake Crystal Structure Of Dry Ice crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. crystal structure of ice ih. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. Plane wave cutoffs of 160 and 960 ry were used for. almost all ice is crystalline,. Crystal Structure Of Dry Ice.
From www.benbest.com
LESSONS FOR CRYONICS FROM METALLURGY AND CERAMICS Crystal Structure Of Dry Ice The equilibrium structure to which a material crystallizes under given conditions of temperature and. crystal structure of ice ih. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. Plane wave cutoffs of 160 and 960 ry were used for. a classic study found that crystalline ice adopts an. Crystal Structure Of Dry Ice.
From www.alamy.com
Ice crystal, structure, black and white image Stock Photo Alamy Crystal Structure Of Dry Ice crystal structure of ice ih. Plane wave cutoffs of 160 and 960 ry were used for. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. a classic study found that crystalline ice adopts an amorphous form when compressed. Two of. Crystal Structure Of Dry Ice.
From www.sciencephoto.com
Ice lattice Stock Image F003/9101 Science Photo Library Crystal Structure Of Dry Ice a classic study found that crystalline ice adopts an amorphous form when compressed. Plane wave cutoffs of 160 and 960 ry were used for. crystal structure of ice ih. crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. almost all ice is crystalline, and much natural ice is. Crystal Structure Of Dry Ice.
From www.slideserve.com
PPT Crystal Structure of Ice PowerPoint Presentation, free Crystal Structure Of Dry Ice In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. Plane wave cutoffs of 160 and 960 ry were used for. Experiments now find that alternative phase. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be. Crystal Structure Of Dry Ice.
From www.sciencephoto.com
Molecular Model of Ice Stock Image A602/0104 Science Photo Library Crystal Structure Of Dry Ice almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. Experiments now find that alternative phase. crystal structure of ice ih. a classic study found that crystalline ice adopts an amorphous form when compressed. In the model we can clearly see. Crystal Structure Of Dry Ice.
From www.alamy.com
crystal of snow, ice crystal, microscopic photograph Stock Photo Alamy Crystal Structure Of Dry Ice crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. crystal structure of ice ih. In the model we can clearly see that each. Crystal Structure Of Dry Ice.
From commons.wikimedia.org
FileIce XI View along c axis.png Wikimedia Commons Crystal Structure Of Dry Ice a classic study found that crystalline ice adopts an amorphous form when compressed. Experiments now find that alternative phase. crystal structure of ice ih. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. The equilibrium structure to which a material crystallizes under given conditions of temperature and. . Crystal Structure Of Dry Ice.
From serc.carleton.edu
3C Make A Glacier Crystal Structure Of Dry Ice crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. The equilibrium structure to which a material crystallizes under given conditions of temperature and. Plane wave cutoffs of 160 and 960 ry were used for. a classic study found that crystalline ice adopts an amorphous form when compressed. almost all. Crystal Structure Of Dry Ice.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 Crystal Structure Of Dry Ice crystal structure of ice ih. Experiments now find that alternative phase. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. Plane wave cutoffs of 160 and 960 ry were used for. The equilibrium structure to which a material crystallizes under given conditions of temperature and. a classic study. Crystal Structure Of Dry Ice.
From www.researchgate.net
The crystal structure of the unit cell of cubic CO 2 I. Carbon and Crystal Structure Of Dry Ice Experiments now find that alternative phase. Plane wave cutoffs of 160 and 960 ry were used for. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. The equilibrium structure to which a material crystallizes under given conditions of temperature and. crystalline dry ice has a cubic symmetry with the. Crystal Structure Of Dry Ice.
From mungfali.com
Dry Ice Structure Crystal Structure Of Dry Ice Plane wave cutoffs of 160 and 960 ry were used for. The equilibrium structure to which a material crystallizes under given conditions of temperature and. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. a classic study found that crystalline ice adopts an amorphous form when compressed. crystalline. Crystal Structure Of Dry Ice.
From shaunmwilliams.com
Lecture 10 Presentation Crystal Structure Of Dry Ice In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. crystal structure of ice ih. The equilibrium structure to which a material crystallizes under given conditions of temperature and. a. Crystal Structure Of Dry Ice.
From www.mdpi.com
Molecules Free FullText Phase Transition of Ice at High Pressures Crystal Structure Of Dry Ice crystal structure of ice ih. Experiments now find that alternative phase. a classic study found that crystalline ice adopts an amorphous form when compressed. Plane wave cutoffs of 160 and 960 ry were used for. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. Two of these are. Crystal Structure Of Dry Ice.
From www.alamy.de
Schnee, Kristalle, Natur, Form, Struktur, Eis, Kälte, Schnee, Kristall Crystal Structure Of Dry Ice The equilibrium structure to which a material crystallizes under given conditions of temperature and. Plane wave cutoffs of 160 and 960 ry were used for. crystal structure of ice ih. Experiments now find that alternative phase. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. In the model we. Crystal Structure Of Dry Ice.
From melscience.com
How to make dry ice MEL Chemistry Crystal Structure Of Dry Ice almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. a classic study found that crystalline ice adopts an amorphous form when compressed. crystal structure of ice ih. In the model we can clearly see that each o atom is surrounded. Crystal Structure Of Dry Ice.
From ar.inspiredpencil.com
Ice Molecule Crystal Structure Of Dry Ice Plane wave cutoffs of 160 and 960 ry were used for. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. crystalline dry. Crystal Structure Of Dry Ice.
From www.heartofavalonia.org
PropertiesIce Crystal Structure Of Dry Ice almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. a classic study found that crystalline ice adopts an amorphous form when compressed. Plane wave cutoffs of 160 and 960 ry were used for. The equilibrium structure to which a material crystallizes. Crystal Structure Of Dry Ice.
From www.chemtube3d.com
Ice water in the solid state Crystal Structure Of Dry Ice almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. crystalline dry ice has a cubic symmetry with the space group of p. Crystal Structure Of Dry Ice.
From www.mtg.msm.cam.ac.uk
Two Dimensional Ice from First Principles Structures and Phase Crystal Structure Of Dry Ice crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. The equilibrium structure to which a material crystallizes under given conditions of temperature and. a classic study found that crystalline ice adopts an amorphous form when compressed. almost all ice is crystalline, and much natural ice is monocrystalline, yet single. Crystal Structure Of Dry Ice.
From www.agsalt.com
How Ice Melt Works to Melt Ice AgSalt Processing, LLC Crystal Structure Of Dry Ice The equilibrium structure to which a material crystallizes under given conditions of temperature and. Plane wave cutoffs of 160 and 960 ry were used for. Experiments now find that alternative phase. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. a. Crystal Structure Of Dry Ice.
From www.sciencephoto.com
Molecular model of ice Stock Image A504/0137 Science Photo Library Crystal Structure Of Dry Ice In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. a classic study found that crystalline ice adopts an amorphous form when compressed.. Crystal Structure Of Dry Ice.
From www.indigoinstruments.com
Unit Ice Crystal Lattice Model Crystal Structure Of Dry Ice almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. In the model we can clearly see that each o atom is surrounded by. Crystal Structure Of Dry Ice.
From www.sciencephoto.com
Molecular structure of ice Stock Image A504/0062 Science Photo Crystal Structure Of Dry Ice crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. crystal structure of ice ih. Two of these are at a distance of 99 pm and are clearly covalently bonded to the. Crystal Structure Of Dry Ice.
From www.youtube.com
What is the chemical formula of dry ice ? QnA Explained YouTube Crystal Structure Of Dry Ice The equilibrium structure to which a material crystallizes under given conditions of temperature and. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. crystal structure of ice ih. crystalline dry ice has a cubic symmetry with the space group of. Crystal Structure Of Dry Ice.
From www.reddit.com
Can carbon dioxide crystallize? askscience Crystal Structure Of Dry Ice Experiments now find that alternative phase. The equilibrium structure to which a material crystallizes under given conditions of temperature and. crystal structure of ice ih. a classic study found that crystalline ice adopts an amorphous form when compressed. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. . Crystal Structure Of Dry Ice.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Crystal Structure Of Dry Ice almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to be useful to the. Experiments now find that alternative phase. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. Plane wave cutoffs of 160 and 960 ry. Crystal Structure Of Dry Ice.
From cissink24.de
Dry Ice Structure Crystal Structure Of Dry Ice In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. Two of these are at a distance of 99 pm and are clearly covalently bonded to the o atom. The equilibrium structure to which a material crystallizes under given conditions of temperature and. Experiments now find that alternative phase. almost. Crystal Structure Of Dry Ice.
From www.pnas.org
Atomic distribution and local structure in ice VII from in situ neutron Crystal Structure Of Dry Ice In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. almost all ice is crystalline, and much natural ice is monocrystalline, yet single crystals of ice are seldom well enough developed to. Crystal Structure Of Dry Ice.
From www.sciencephoto.com
Ice, molecular model Stock Image C002/9165 Science Photo Library Crystal Structure Of Dry Ice Plane wave cutoffs of 160 and 960 ry were used for. crystal structure of ice ih. In the model we can clearly see that each o atom is surrounded by four h atoms arranged tetrahedrally. crystalline dry ice has a cubic symmetry with the space group of p a 3 (see fig. almost all ice is crystalline,. Crystal Structure Of Dry Ice.