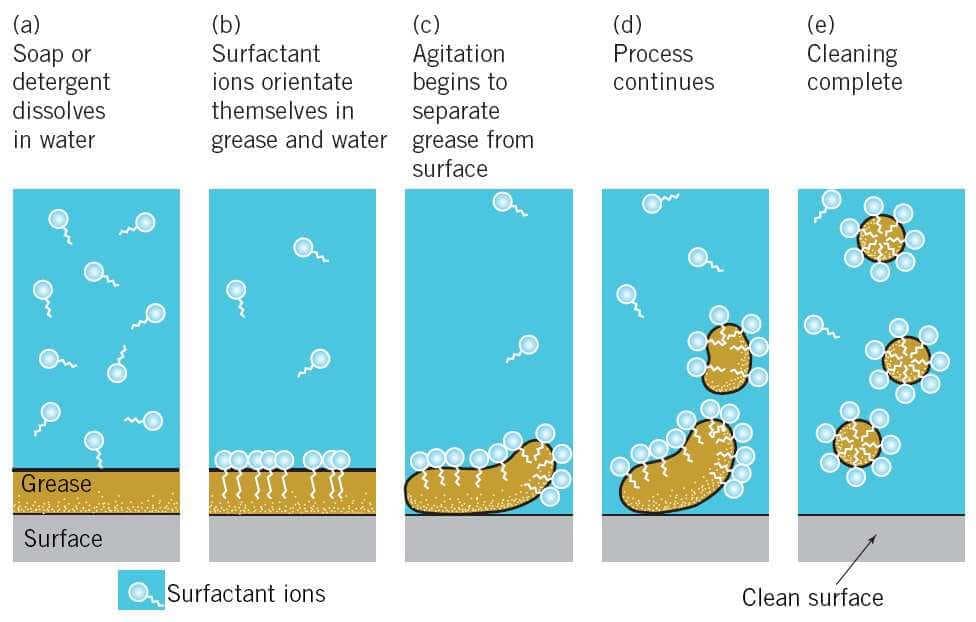
How Does Soap Work Chemistry . The micelle is important because it is what traps the soil. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Soap mixing with oil under a microscope, forming micelles. The oldest amphiphilic cleaning agent known to humans is soap. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. Remember, the inside of the micelle is hydrophobic and does not want to be near water. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is able to clean hands and dishes because of some pretty nifty chemistry. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Find out the difference between soap and. Learn more about soap and detergent in this article. Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension.
from edurev.in
Soap mixing with oil under a microscope, forming micelles. Learn more about soap and detergent in this article. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Remember, the inside of the micelle is hydrophobic and does not want to be near water. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. Find out the difference between soap and. The oldest amphiphilic cleaning agent known to humans is soap. The micelle is important because it is what traps the soil. Soap is able to clean hands and dishes because of some pretty nifty chemistry.
Cleansing Action of Soaps and Detergents Class 10 Notes EduRev
How Does Soap Work Chemistry Soap is able to clean hands and dishes because of some pretty nifty chemistry. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Learn more about soap and detergent in this article. Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Find out the difference between soap and. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Soap mixing with oil under a microscope, forming micelles. The oldest amphiphilic cleaning agent known to humans is soap. Remember, the inside of the micelle is hydrophobic and does not want to be near water. The micelle is important because it is what traps the soil.
From www.youtube.com
How does Soap Work? YouTube How Does Soap Work Chemistry Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The micelle is important because it is what traps the soil. Before sodium hydroxide was commercially available,. How Does Soap Work Chemistry.
From cosmosmagazine.com
The chemistry of soap How Does Soap Work Chemistry Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Soap is able to clean hands and dishes because of some pretty nifty chemistry. The micelle is important because it is what traps the soil. Find out the difference between soap and. Soap mixing with oil under a microscope, forming micelles. Soap. How Does Soap Work Chemistry.
From www.slideserve.com
PPT Preparation and Properties of a Soap PowerPoint Presentation How Does Soap Work Chemistry Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. Find out the difference between soap and. The oldest amphiphilic cleaning agent known to humans is soap. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as. How Does Soap Work Chemistry.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Chemistry Soap mixing with oil under a microscope, forming micelles. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Learn more about soap and detergent in this article. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Find out the difference between. How Does Soap Work Chemistry.
From newtondesk.com
Why Are Bubbles Formed In Soap Solution? Types of Soap How Does Soap Work Chemistry The oldest amphiphilic cleaning agent known to humans is soap. The micelle is important because it is what traps the soil. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Learn how. How Does Soap Work Chemistry.
From www.tffn.net
The Chemistry of Soap How Does it Work? The Enlightened Mindset How Does Soap Work Chemistry Learn more about soap and detergent in this article. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. Find out the difference between soap and.. How Does Soap Work Chemistry.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Chemistry Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. The oldest amphiphilic cleaning agent known to humans is soap. Soap mixing with oil under a microscope, forming micelles. Learn more about soap and detergent in this article. Soap is able to clean hands and dishes because of some pretty nifty chemistry.. How Does Soap Work Chemistry.
From ar.inspiredpencil.com
Soap Molecule Structure How Does Soap Work Chemistry Soap mixing with oil under a microscope, forming micelles. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. Remember, the inside of the micelle is hydrophobic and does not want to be near water. Before sodium hydroxide was commercially available, a boiling solution of. How Does Soap Work Chemistry.
From www.slideserve.com
PPT FUNDAMENTALS OF CHEMISTRY PowerPoint Presentation, free download How Does Soap Work Chemistry The seemingly simple process of cleaning a soiled surface is, in fact, complex. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Find out the difference between soap and. The oldest amphiphilic cleaning agent known to humans is soap. Soap and detergent, substances that, when dissolved in water, possess the. How Does Soap Work Chemistry.
From www.defeatdd.org
How does soap actually work? How Does Soap Work Chemistry Soap mixing with oil under a microscope, forming micelles. Learn more about soap and detergent in this article. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. How Does Soap Work Chemistry.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Chemistry The oldest amphiphilic cleaning agent known to humans is soap. The micelle is important because it is what traps the soil. Remember, the inside of the micelle is hydrophobic and does not want to be near water. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Soap is able to clean hands and dishes because of. How Does Soap Work Chemistry.
From www.theodysseyonline.com
How Does Soap Work? How Does Soap Work Chemistry Soap mixing with oil under a microscope, forming micelles. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other. How Does Soap Work Chemistry.
From www.pinterest.com
Cleansing Action Of Soap. Soap, Cleanse, Molecules How Does Soap Work Chemistry Soap mixing with oil under a microscope, forming micelles. Remember, the inside of the micelle is hydrophobic and does not want to be near water. Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. The seemingly simple process of cleaning a soiled surface is, in. How Does Soap Work Chemistry.
From www.slideserve.com
PPT HOW SOAP WORKS PowerPoint Presentation, free download ID1114540 How Does Soap Work Chemistry The seemingly simple process of cleaning a soiled surface is, in fact, complex. The micelle is important because it is what traps the soil. Learn more about soap and detergent in this article. Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. The oldest amphiphilic. How Does Soap Work Chemistry.
From www.slideserve.com
PPT Preparation and Properties of a Soap PowerPoint Presentation How Does Soap Work Chemistry Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. How Does Soap Work Chemistry.
From www.themacbath.com
Back to Basics What Is Soap and How Does It Work? — The MacBath How Does Soap Work Chemistry Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Learn more about soap and detergent in this article. Soap mixing with oil under a microscope, forming micelles.. How Does Soap Work Chemistry.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Chemistry Remember, the inside of the micelle is hydrophobic and does not want to be near water. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. The seemingly simple process of cleaning a soiled surface is, in fact, complex. The micelle is important because it is what traps the soil. Before. How Does Soap Work Chemistry.
From in.pinterest.com
Hand washing with soap vector illustration. Educational explanation How Does Soap Work Chemistry Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Soap is able. How Does Soap Work Chemistry.
From www.thoughtco.com
How Soap Works How Does Soap Work Chemistry Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. Soap mixing with oil under a microscope, forming micelles. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached. How Does Soap Work Chemistry.
From www.meritech.com
How Does Soap Work? How Soap Works to Remove Germs and Pathogens How Does Soap Work Chemistry Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Soap is able to. How Does Soap Work Chemistry.
From www.thebodybean.com
How does Soap work? — The Body Bean How Does Soap Work Chemistry Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Find out the difference between soap and. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Remember, the inside of the micelle is hydrophobic and does not want to be near water. The seemingly simple process. How Does Soap Work Chemistry.
From www.freepik.com
Premium Vector How soap works vector illustration infographic How Does Soap Work Chemistry The micelle is important because it is what traps the soil. Soap mixing with oil under a microscope, forming micelles. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Remember, the inside of the micelle is hydrophobic and does not want to be near water. Find out the difference between soap and. Soap and detergent,. How Does Soap Work Chemistry.
From www.pinterest.com
How Soap Work? Soap, Cleanse, Basic concepts How Does Soap Work Chemistry Soap is able to clean hands and dishes because of some pretty nifty chemistry. Remember, the inside of the micelle is hydrophobic and does not want to be near water. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. Learn how soap's unique chemistry. How Does Soap Work Chemistry.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Chemistry Learn more about soap and detergent in this article. Soap molecules have on one end what’s known as a polar salt, which is hydrophilic, or attracted to water. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Soap is able to clean hands and dishes because of some pretty nifty chemistry.. How Does Soap Work Chemistry.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 How Does Soap Work Chemistry Find out the difference between soap and. The oldest amphiphilic cleaning agent known to humans is soap. The micelle is important because it is what traps the soil. Soap mixing with oil under a microscope, forming micelles. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and. How Does Soap Work Chemistry.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Chemistry Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. Soap is able to clean hands and dishes because of some pretty nifty chemistry. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. The oldest amphiphilic cleaning. How Does Soap Work Chemistry.
From www.youtube.com
Chemistry 101 How does soap work? YouTube How Does Soap Work Chemistry Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. Learn more about soap and detergent in this article. Soap is. How Does Soap Work Chemistry.
From www.meritech.com
How Does Soap Work? How Soap Works to Remove Germs and Pathogens How Does Soap Work Chemistry Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. Soap mixing with oil under a microscope, forming micelles. The micelle is important because it is what traps the soil. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Find out the. How Does Soap Work Chemistry.
From www.slideshare.net
Chemistry of soaps How Does Soap Work Chemistry Find out the difference between soap and. Soap mixing with oil under a microscope, forming micelles. The micelle is important because it is what traps the soil. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. The oldest amphiphilic cleaning agent known to humans is soap. The seemingly simple process of. How Does Soap Work Chemistry.
From www.youtube.com
How does soap work? YouTube How Does Soap Work Chemistry Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. Remember, the inside of the micelle is hydrophobic and does not want to be near water. Learn. How Does Soap Work Chemistry.
From edurev.in
Cleansing Action of Soaps and Detergents Class 10 Notes EduRev How Does Soap Work Chemistry The oldest amphiphilic cleaning agent known to humans is soap. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Remember, the inside of the micelle is hydrophobic and does not want to be near water. Learn more about soap and detergent in this article. Soap is able to clean hands and dishes because of some pretty. How Does Soap Work Chemistry.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Chemistry Soap mixing with oil under a microscope, forming micelles. Before sodium hydroxide was commercially available, a boiling solution of potassium carbonate leached from wood ashes was used. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Soap is able to clean hands and dishes because of some pretty nifty chemistry. The oldest amphiphilic cleaning agent known. How Does Soap Work Chemistry.
From www.slideserve.com
PPT How Does Soap Work? PowerPoint Presentation, free download ID How Does Soap Work Chemistry Find out the difference between soap and. The micelle is important because it is what traps the soil. Remember, the inside of the micelle is hydrophobic and does not want to be near water. The seemingly simple process of cleaning a soiled surface is, in fact, complex. Learn more about soap and detergent in this article. Before sodium hydroxide was. How Does Soap Work Chemistry.
From www.slideserve.com
PPT HOW SOAP WORKS PowerPoint Presentation, free download ID1114540 How Does Soap Work Chemistry Learn more about soap and detergent in this article. Remember, the inside of the micelle is hydrophobic and does not want to be near water. Learn how soap's unique chemistry allows it to dissolve in both water and oil, and how it can clean surfaces and decrease surface tension. Soap molecules have on one end what’s known as a polar. How Does Soap Work Chemistry.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents How Does Soap Work Chemistry Soap is able to clean hands and dishes because of some pretty nifty chemistry. Learn more about soap and detergent in this article. The micelle is important because it is what traps the soil. Soap and detergent, substances that, when dissolved in water, possess the ability to remove dirt from surfaces such as human skin, textiles, and other solids. Remember,. How Does Soap Work Chemistry.