Copper And Zinc Cell . This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The charge is balanced because the 2+ charge on the. The picture opposite shows a galvanic cell made from zinc and tin half cells. a galvanic cell contains two compartments: zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The reactions occurring are those. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The zinc half cell has a strip.
from byjus.com
The picture opposite shows a galvanic cell made from zinc and tin half cells. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The reactions occurring are those. The charge is balanced because the 2+ charge on the. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). a galvanic cell contains two compartments: The zinc half cell has a strip.
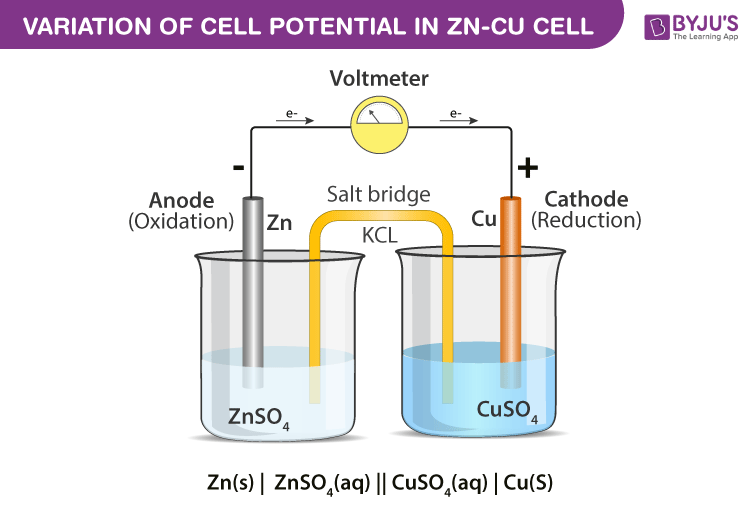
Variation of Cell Potential in ZnCu Cell Chemistry Practicals Class
Copper And Zinc Cell use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The picture opposite shows a galvanic cell made from zinc and tin half cells. a galvanic cell contains two compartments: The charge is balanced because the 2+ charge on the. The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The zinc half cell has a strip.
From colouremployer8.gitlab.io
Marvelous Copper Zinc Battery Reaction Cie Chemistry A Level Syllabus Copper And Zinc Cell use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. a galvanic cell contains two compartments: This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The charge is balanced because the 2+ charge on the. The reactions occurring are those. The picture opposite shows a galvanic. Copper And Zinc Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Copper And Zinc Cell The zinc half cell has a strip. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The picture opposite shows a galvanic cell made from zinc and tin half cells. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc.. Copper And Zinc Cell.
From www.alamy.com
Daniell element galvanic cell with zinc and copper Stock Photo Copper And Zinc Cell The charge is balanced because the 2+ charge on the. a galvanic cell contains two compartments: This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The zinc half cell has a strip. The reactions occurring are. Copper And Zinc Cell.
From www.alamy.com
Copper zinc cell Cut Out Stock Images & Pictures Alamy Copper And Zinc Cell The zinc half cell has a strip. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The picture opposite shows a galvanic cell made from zinc and tin half cells. The charge is balanced because the 2+ charge on the. The reactions occurring are those. This 2:54 minute video shows. Copper And Zinc Cell.
From www.youtube.com
ZincCopper Electrochemical Cell Demonstration YouTube Copper And Zinc Cell The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). a galvanic cell contains two compartments: The charge is balanced because the 2+ charge on the. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and. Copper And Zinc Cell.
From integrated-mcat.com
Science Image Archive for Teachers Copper And Zinc Cell This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The charge is balanced because the 2+ charge on the. The reactions occurring are those. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. a galvanic cell contains two compartments: The zinc half cell has a. Copper And Zinc Cell.
From www.researchgate.net
Hector PEREZ Doctor of Philosophy Engineering University of Copper And Zinc Cell use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). a galvanic cell contains two compartments: The reactions occurring are those. The picture opposite shows a galvanic cell made from. Copper And Zinc Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2810901 Copper And Zinc Cell The zinc half cell has a strip. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The charge is balanced because the 2+ charge on the. use cell notation to. Copper And Zinc Cell.
From mavink.com
Zinc Copper Galvanic Cell Copper And Zinc Cell The charge is balanced because the 2+ charge on the. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The reactions occurring are those. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. This 2:54 minute video shows the. Copper And Zinc Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID1195562 Copper And Zinc Cell a galvanic cell contains two compartments: use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The zinc half cell has a strip. The picture opposite shows a galvanic cell made from zinc and tin half cells. The reactions occurring are those. zinc behaves as the anode (supplying electrons). Copper And Zinc Cell.
From www1.chem.umn.edu
CopperZinc Galvanic Cell Copper And Zinc Cell This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The picture opposite shows a galvanic cell made from zinc and tin half cells. The charge is balanced because the 2+ charge on the. The reactions occurring are those. a galvanic cell contains two compartments: The zinc half cell has a strip. use cell notation. Copper And Zinc Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Copper And Zinc Cell The charge is balanced because the 2+ charge on the. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). This 2:54 minute video shows the. Copper And Zinc Cell.
From www.freepik.com
Premium Photo Electrochemical cell or Galvanic cell. The Daniell cell Copper And Zinc Cell The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The zinc half cell has a strip. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to. Copper And Zinc Cell.
From blog.thepipingmart.com
Classifications of Metals Copper vs. Zinc Copper And Zinc Cell use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The charge is balanced because the 2+ charge. Copper And Zinc Cell.
From www.slideserve.com
PPT Chapter Twenty PowerPoint Presentation, free download ID5368420 Copper And Zinc Cell This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The zinc half cell has a strip. The picture opposite shows a galvanic cell made from zinc and tin half cells. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The charge is balanced because the 2+. Copper And Zinc Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5405206 Copper And Zinc Cell zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The zinc half cell has a strip. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The. Copper And Zinc Cell.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Copper And Zinc Cell zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The charge is balanced because the 2+ charge on the. a galvanic cell contains two compartments: The picture opposite shows a galvanic cell made from zinc and tin half cells. The reactions occurring are those. This 2:54 minute video. Copper And Zinc Cell.
From quizlet.com
Label the zinc/copper galvanic cell Diagram Quizlet Copper And Zinc Cell zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The picture opposite shows a galvanic cell made from zinc and tin half cells. The charge is balanced because the 2+ charge on the. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper. Copper And Zinc Cell.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Copper And Zinc Cell zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The picture opposite shows a galvanic cell made. Copper And Zinc Cell.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Copper And Zinc Cell a galvanic cell contains two compartments: The charge is balanced because the 2+ charge on the. The picture opposite shows a galvanic cell made from zinc and tin half cells. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. This 2:54 minute video shows the spontaneous reaction between copper. Copper And Zinc Cell.
From ar.inspiredpencil.com
Copper Electrolytic Cell Copper And Zinc Cell The reactions occurring are those. The picture opposite shows a galvanic cell made from zinc and tin half cells. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The charge is balanced because the 2+ charge on the. The zinc half cell has a strip. This 2:54 minute video. Copper And Zinc Cell.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Copper And Zinc Cell use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The picture opposite shows a galvanic cell made from zinc and tin half cells. The zinc half cell has a strip. The charge is balanced because the 2+ charge on the. zinc behaves as the anode (supplying electrons) of the. Copper And Zinc Cell.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Copper And Zinc Cell use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The picture opposite shows a galvanic cell made from zinc and tin half cells. a galvanic cell contains two compartments: The reactions occurring are those. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The charge. Copper And Zinc Cell.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram Copper And Zinc Cell The picture opposite shows a galvanic cell made from zinc and tin half cells. The charge is balanced because the 2+ charge on the. The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). a galvanic cell contains two compartments: This 2:54 minute video. Copper And Zinc Cell.
From www.slideserve.com
PPT What is an Electrochemical Cell? PowerPoint Presentation, free Copper And Zinc Cell The picture opposite shows a galvanic cell made from zinc and tin half cells. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The zinc half cell has a strip. a galvanic cell contains two compartments: The charge is balanced because the 2+ charge on the. use. Copper And Zinc Cell.
From www.coursehero.com
[Solved] Sketch a diagram of a copper/zinc Daniell cell. Label all the Copper And Zinc Cell use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The zinc half cell has a strip. The charge is balanced because the 2+ charge on the. The picture opposite shows. Copper And Zinc Cell.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Copper And Zinc Cell a galvanic cell contains two compartments: The picture opposite shows a galvanic cell made from zinc and tin half cells. The reactions occurring are those. The charge is balanced because the 2+ charge on the. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). This 2:54 minute video. Copper And Zinc Cell.
From byjus.com
Variation of Cell Potential in ZnCu Cell Chemistry Practicals Class Copper And Zinc Cell The picture opposite shows a galvanic cell made from zinc and tin half cells. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The charge is balanced because the 2+ charge on the. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The reactions occurring are. Copper And Zinc Cell.
From cider.uoregon.edu
Electrochemcial Cell Demonstration Voltaic Cell Zinc/Copper CIDER Copper And Zinc Cell use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). a galvanic cell contains two compartments: The. Copper And Zinc Cell.
From brainly.in
label the parts of simple electrical cell (diagram given above Copper And Zinc Cell The charge is balanced because the 2+ charge on the. The reactions occurring are those. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The zinc half cell has a strip. The picture opposite shows a galvanic cell made from zinc and tin half cells. a galvanic cell contains two compartments: zinc behaves as. Copper And Zinc Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1937020 Copper And Zinc Cell use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The zinc half cell has a strip. a galvanic cell contains two compartments: This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The charge is balanced because the 2+ charge on the. The reactions occurring are. Copper And Zinc Cell.
From userdatarheumatics.z21.web.core.windows.net
Simple Cell Diagram Chemistry Copper And Zinc Cell a galvanic cell contains two compartments: The reactions occurring are those. The zinc half cell has a strip. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The charge is balanced because the 2+ charge on the. This 2:54 minute video shows the spontaneous reaction between copper ions. Copper And Zinc Cell.
From www.slideserve.com
PPT Chapter 22 Electrochemistry PowerPoint Presentation, free Copper And Zinc Cell zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The reactions occurring are those. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. a galvanic cell contains two compartments: use cell notation to describe the galvanic cell where copper(ii) ions are reduced to. Copper And Zinc Cell.
From dxossebxk.blob.core.windows.net
Copper Ore Extraction at Lewis Johnson blog Copper And Zinc Cell This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper as the cathode (consuming electrons). The zinc half cell has a strip. . Copper And Zinc Cell.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Copper And Zinc Cell The picture opposite shows a galvanic cell made from zinc and tin half cells. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. The zinc half cell has a strip. The reactions occurring are those. zinc behaves as the anode (supplying electrons) of the galvanic cell and the copper. Copper And Zinc Cell.