How To Insulate A Calorimeter . you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. One technique we can use to measure the amount of heat involved in a chemical or physical. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. measuring heat flow. the task and the engineering cycle. The fiber ring is made of insulating fiber material.
from users.highland.edu
One technique we can use to measure the amount of heat involved in a chemical or physical. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. The fiber ring is made of insulating fiber material. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. the task and the engineering cycle. calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). measuring heat flow. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two.
Calorimetry
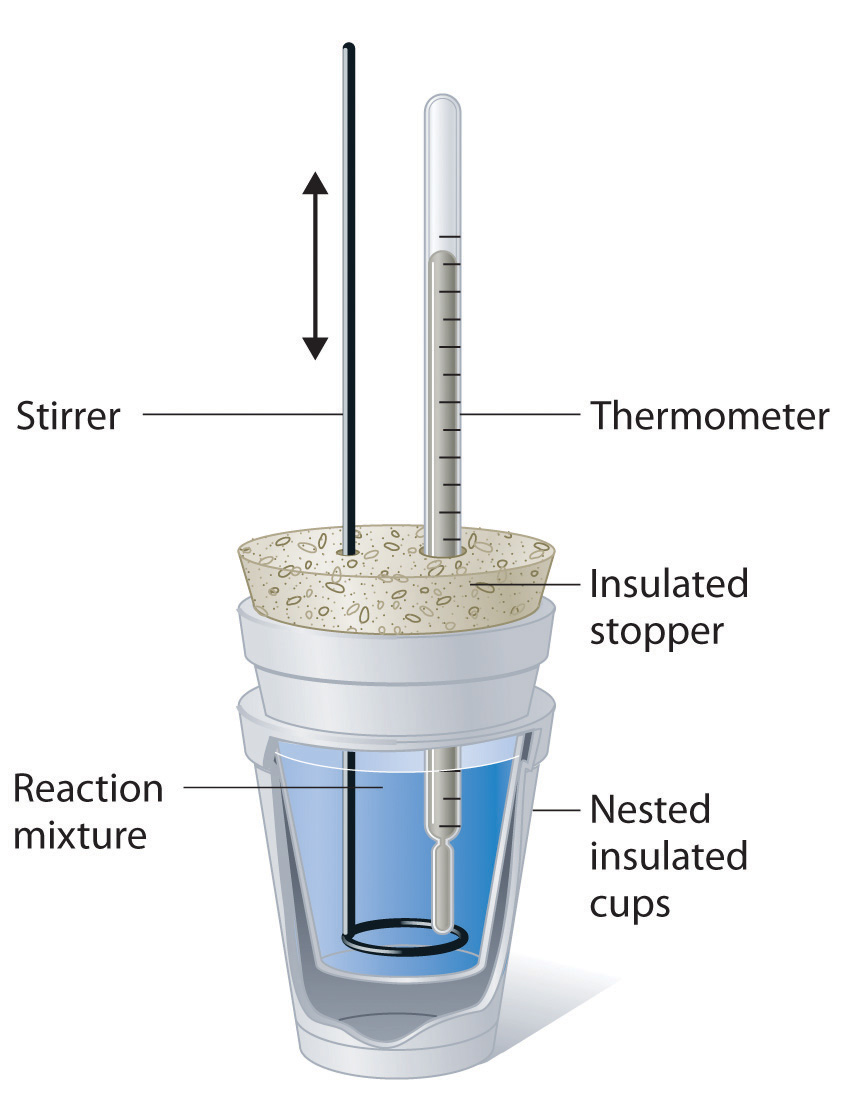
How To Insulate A Calorimeter One technique we can use to measure the amount of heat involved in a chemical or physical. measuring heat flow. The fiber ring is made of insulating fiber material. One technique we can use to measure the amount of heat involved in a chemical or physical. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. the task and the engineering cycle. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep How To Insulate A Calorimeter The fiber ring is made of insulating fiber material. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). you can correct for the effective heat capacity of the. How To Insulate A Calorimeter.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure How To Insulate A Calorimeter measuring heat flow. The fiber ring is made of insulating fiber material. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8. How To Insulate A Calorimeter.
From exoarnnpu.blob.core.windows.net
Calorimeter Description And Function at Kyla Ochs blog How To Insulate A Calorimeter you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). measuring heat flow. the task and the engineering cycle. this calorimeter consists of an outer (127 mm height x 101 mm diameter). How To Insulate A Calorimeter.
From www.researchgate.net
Calorimeter with the insulation cover. Download Scientific Diagram How To Insulate A Calorimeter The fiber ring is made of insulating fiber material. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. One technique we can use to measure the amount of heat involved in a chemical or physical. calorimeters have a fiber ring that hold the inner. How To Insulate A Calorimeter.
From psu.pb.unizin.org
Calorimetry (9.2) Chemistry 110 How To Insulate A Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. The fiber ring is made of insulating fiber material. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). the task and the engineering cycle. One technique we can use to measure the amount of heat involved in a chemical or physical. . How To Insulate A Calorimeter.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog How To Insulate A Calorimeter this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimeters have a fiber ring that hold the inner vessel hanging in the center of. How To Insulate A Calorimeter.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica How To Insulate A Calorimeter One technique we can use to measure the amount of heat involved in a chemical or physical. the task and the engineering cycle. measuring heat flow. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. Calorimeters are thermally insulated to minimize the heat. How To Insulate A Calorimeter.
From socratic.org
What is the term used for a well insulated reaction vessel used to How To Insulate A Calorimeter One technique we can use to measure the amount of heat involved in a chemical or physical. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). calculate heat,. How To Insulate A Calorimeter.
From www.slideserve.com
PPT Hot Packs and Cold Packs PowerPoint Presentation, free download How To Insulate A Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. measuring heat flow. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). The fiber ring is made of insulating fiber material. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height. How To Insulate A Calorimeter.
From www.thetechedvocate.org
How to Build a Calorimeter The Tech Edvocate How To Insulate A Calorimeter this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. the task and the engineering cycle. measuring heat flow. The fiber ring is made of insulating fiber material. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows).. How To Insulate A Calorimeter.
From thermonine92.blogspot.com
Thermochemistry Calorimeter How To Insulate A Calorimeter this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. One technique we can use to measure the amount of heat involved in a chemical or. How To Insulate A Calorimeter.
From chemistry.stackexchange.com
thermodynamics how to improve selfconstructed calorimeter (heat How To Insulate A Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. One technique we can use to measure the amount of heat involved in a chemical or. How To Insulate A Calorimeter.
From users.highland.edu
Calorimetry How To Insulate A Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. One technique we can use to measure the amount of heat involved in a chemical or physical. measuring heat flow. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by.. How To Insulate A Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How To Insulate A Calorimeter One technique we can use to measure the amount of heat involved in a chemical or physical. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). The fiber ring is made of insulating fiber material. measuring heat flow. the task and the engineering cycle. calculate heat, temperature change, and specific heat after thermal equilibrium is. How To Insulate A Calorimeter.
From pathwaystochemistry.com
Calorimetry Pathways to Chemistry How To Insulate A Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. calorimeters have a fiber ring that hold the inner vessel hanging in the center of. How To Insulate A Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How To Insulate A Calorimeter this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. measuring heat flow. calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. calculate heat, temperature change, and specific heat. How To Insulate A Calorimeter.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness How To Insulate A Calorimeter One technique we can use to measure the amount of heat involved in a chemical or physical. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. How To Insulate A Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry I How To Insulate A Calorimeter measuring heat flow. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. One technique we can use to measure the amount of heat involved in a chemical or physical. calorimeters have a fiber ring that hold the inner. How To Insulate A Calorimeter.
From www.animalia-life.club
Calorimeter Diagram How To Insulate A Calorimeter the task and the engineering cycle. The fiber ring is made of insulating fiber material. One technique we can use to measure the amount of heat involved in a chemical or physical. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. Calorimeters are thermally. How To Insulate A Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry How To Insulate A Calorimeter measuring heat flow. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). The fiber ring is made of insulating fiber material. One technique we can use to measure the amount of heat involved in a chemical or physical. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before. How To Insulate A Calorimeter.
From www.slideshare.net
Tang 01 heat capacity and calorimetry How To Insulate A Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. the task and the engineering cycle. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). this calorimeter consists of an outer (127. How To Insulate A Calorimeter.
From exoxbgvei.blob.core.windows.net
Calorimeter Laboratory at Clara Carter blog How To Insulate A Calorimeter calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. One technique we can use to measure the amount of heat involved in a chemical or physical. measuring heat flow. The fiber ring is made of insulating fiber material. this calorimeter consists of an outer (127 mm height x. How To Insulate A Calorimeter.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture How To Insulate A Calorimeter calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. One technique we can use to measure the amount of heat involved in a chemical or physical. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). measuring heat flow. you can correct for the effective heat capacity. How To Insulate A Calorimeter.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube How To Insulate A Calorimeter The fiber ring is made of insulating fiber material. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. One technique we can use to measure the amount of heat involved in a chemical or physical. calorimeters have a fiber ring that hold the inner. How To Insulate A Calorimeter.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle How To Insulate A Calorimeter Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. measuring heat flow. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an. How To Insulate A Calorimeter.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME How To Insulate A Calorimeter One technique we can use to measure the amount of heat involved in a chemical or physical. The fiber ring is made of insulating fiber material. calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and. How To Insulate A Calorimeter.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas How To Insulate A Calorimeter you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). The fiber ring is made of insulating fiber material. the task and the engineering cycle. measuring heat flow. One technique we can use. How To Insulate A Calorimeter.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer How To Insulate A Calorimeter Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. The fiber ring is made of insulating fiber material. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height. How To Insulate A Calorimeter.
From www.slideserve.com
PPT Chapter 17 “Thermochemistry” PowerPoint Presentation, free How To Insulate A Calorimeter you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. One technique we can use to measure the amount of heat involved in a chemical or physical.. How To Insulate A Calorimeter.
From dxoinieui.blob.core.windows.net
Calorimeter Diagram Class 11 at Ollie Stringer blog How To Insulate A Calorimeter Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). The fiber ring is made of insulating fiber material. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. you can correct for the effective heat capacity of the. How To Insulate A Calorimeter.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson How To Insulate A Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. you can correct for the effective heat capacity of the calorimeter if you characterize it carefully (either before the actual experiment, or by. the task and the engineering cycle. measuring heat flow. calorimeters have a fiber ring that hold the inner. How To Insulate A Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry How To Insulate A Calorimeter One technique we can use to measure the amount of heat involved in a chemical or physical. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. How To Insulate A Calorimeter.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas How To Insulate A Calorimeter the task and the engineering cycle. calorimeters have a fiber ring that hold the inner vessel hanging in the center of the outer vessel. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. measuring heat flow. this calorimeter consists of an outer (127 mm height x 101 mm diameter) and. How To Insulate A Calorimeter.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter How To Insulate A Calorimeter this calorimeter consists of an outer (127 mm height x 101 mm diameter) and an inner part (50.8 mm height x 50.8 diameter), with an insulated space in. One technique we can use to measure the amount of heat involved in a chemical or physical. calorimeters have a fiber ring that hold the inner vessel hanging in the. How To Insulate A Calorimeter.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog How To Insulate A Calorimeter calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. The fiber ring is made of insulating fiber material. One technique we can use to measure the amount of heat involved in a chemical or physical. Calorimeters are thermally insulated to minimize the heat exchange (orange arrows). you can correct for the effective heat. How To Insulate A Calorimeter.