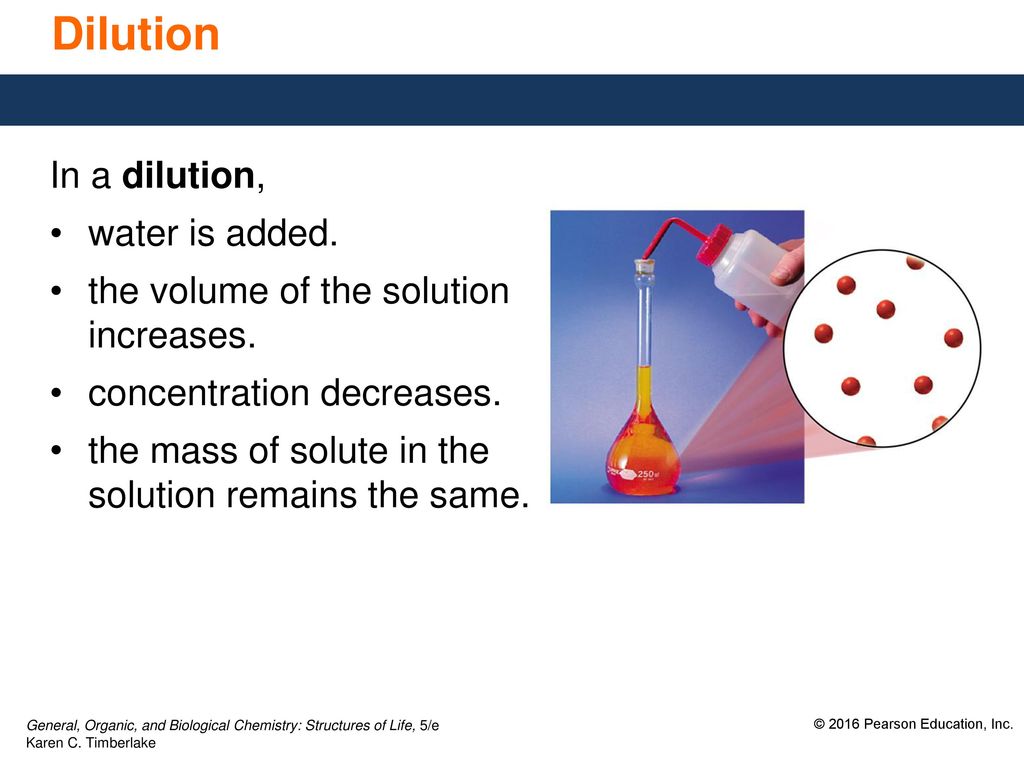
Dilution Of Water By Solute . To dilute a solution means to add more solvent without the addition of more solute. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Of course, the resulting solution is thoroughly mixed so as to. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is a process used to lower the concentration of the original solution by adding solvents. The concentrated solution is known. For example, we might say that a glass. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Suppose that you have \(100. \text{ml}\) of a \(2.0 \: You dilute the solution by adding enough water to. Dilution is the addition of.
from carlosgokeowen.blogspot.com
Dilution is the addition of. To dilute a solution means to add more solvent without the addition of more solute. Suppose that you have \(100. Of course, the resulting solution is thoroughly mixed so as to. For example, we might say that a glass. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Learn how to dilute and concentrate solutions. You dilute the solution by adding enough water to. \text{ml}\) of a \(2.0 \: The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio.
What is Dilution
Dilution Of Water By Solute You dilute the solution by adding enough water to. Suppose that you have \(100. Dilution is the addition of. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. To dilute a solution means to add more solvent without the addition of more solute. Dilution is a process used to lower the concentration of the original solution by adding solvents. \text{ml}\) of a \(2.0 \: The concentrated solution is known. For example, we might say that a glass. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. You dilute the solution by adding enough water to. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Of course, the resulting solution is thoroughly mixed so as to. Learn how to dilute and concentrate solutions.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Of Water By Solute \text{ml}\) of a \(2.0 \: Often, a worker will need to change the concentration of a solution by changing the amount of solvent. To dilute a solution means to add more solvent without the addition of more solute. Dilution is the addition of. The concentrated solution is known. Dilution is the process whereby the concentration of a solution is lessened. Dilution Of Water By Solute.
From slideplayer.com
Solutions Concentration. ppt download Dilution Of Water By Solute You dilute the solution by adding enough water to. \text{ml}\) of a \(2.0 \: The concentrated solution is known. To dilute a solution means to add more solvent without the addition of more solute. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Learn how. Dilution Of Water By Solute.
From slideplayer.com
Molarity and Dilutions ppt download Dilution Of Water By Solute Of course, the resulting solution is thoroughly mixed so as to. Dilution is a process used to lower the concentration of the original solution by adding solvents. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is the process of “lowering the concentration of a solute in a solution by simply. Dilution Of Water By Solute.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilution Of Water By Solute The concentrated solution is known. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. For example, we might say that a glass. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Dilution is a process used to lower the concentration of the. Dilution Of Water By Solute.
From www.slideshare.net
Water and solutions Dilution Of Water By Solute Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass. You dilute the solution by adding enough water to. To dilute a solution means to add more solvent without the addition of more solute. Dilution is the addition of. Dilution is a process used to. Dilution Of Water By Solute.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Dilution Of Water By Solute Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Learn how to dilute and concentrate solutions. For example, we might say that a glass. Dilution is. Dilution Of Water By Solute.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture Dilution Of Water By Solute For example, we might say that a glass. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. \text{ml}\) of a \(2.0 \: Suppose that you have \(100. The concentrated solution is known. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add. Dilution Of Water By Solute.
From www.studypug.com
Introduction to Solution Chemistry Explore Solubility StudyPug Dilution Of Water By Solute Dilution is a process used to lower the concentration of the original solution by adding solvents. For example, we might say that a glass. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is the process whereby the concentration of a solution is lessened. Dilution Of Water By Solute.
From www.wikihow.com
How to Dilute Solutions 8 Steps (with Pictures) wikiHow Dilution Of Water By Solute Of course, the resulting solution is thoroughly mixed so as to. Suppose that you have \(100. Dilution is the addition of. The concentrated solution is known. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. \text{ml}\) of a \(2.0 \: Dilution is a process used to lower the concentration of. Dilution Of Water By Solute.
From www.chegg.com
Solved VISUALIZATION Preparing a Solution by Dilution WATER Dilution Of Water By Solute Dilution is the addition of. You dilute the solution by adding enough water to. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass. Of course, the. Dilution Of Water By Solute.
From studyschooljoanna.z21.web.core.windows.net
Solute In Science Dilution Of Water By Solute To dilute a solution means to add more solvent without the addition of more solute. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Dilution is the addition of. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the. Dilution Of Water By Solute.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilution Of Water By Solute Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. To dilute a solution means to add more solvent without the addition of more solute. Dilution is the addition of. Suppose that you have \(100. Dilution is a process used to lower the concentration of the. Dilution Of Water By Solute.
From www.yaclass.in
Types of solutions Based on the amount of the solute — lesson. Science State Board, Class 10. Dilution Of Water By Solute Dilution is the addition of. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Of course, the resulting solution is thoroughly mixed so as to. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the process of “lowering the. Dilution Of Water By Solute.
From exozlhxkx.blob.core.windows.net
Water Dilute Solution at Faustina Baptist blog Dilution Of Water By Solute To dilute a solution means to add more solvent without the addition of more solute. For example, we might say that a glass. You dilute the solution by adding enough water to. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Suppose that you have \(100. Often, a worker will. Dilution Of Water By Solute.
From www.slideshare.net
Water and solutions Dilution Of Water By Solute Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of. The concentrated solution is known. To dilute a solution means to add more solvent without the addition of more solute. \text{ml}\) of a \(2.0 \: The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio.. Dilution Of Water By Solute.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Art of Science Dilution Of Water By Solute Often, a worker will need to change the concentration of a solution by changing the amount of solvent. \text{ml}\) of a \(2.0 \: Learn how to dilute and concentrate solutions. Suppose that you have \(100. Dilution is a process used to lower the concentration of the original solution by adding solvents. Of course, the resulting solution is thoroughly mixed so. Dilution Of Water By Solute.
From www.slideserve.com
PPT Water and the aqueous systems PowerPoint Presentation, free download ID3459256 Dilution Of Water By Solute Learn how to dilute and concentrate solutions. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Dilution is a process used to lower the concentration of the original solution by adding solvents. For example, we might say that a glass. The dilution ratio calculator tells. Dilution Of Water By Solute.
From www.slideserve.com
PPT What is osmosis? PowerPoint Presentation, free download ID2712925 Dilution Of Water By Solute Learn how to dilute and concentrate solutions. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. For example, we might say that a glass. Of course, the resulting solution is thoroughly mixed so as to. Dilution is a process used to lower the concentration of the original solution by adding. Dilution Of Water By Solute.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Of Water By Solute The concentrated solution is known. \text{ml}\) of a \(2.0 \: To dilute a solution means to add more solvent without the addition of more solute. You dilute the solution by adding enough water to. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions. Dilution. Dilution Of Water By Solute.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Of Water By Solute Dilution is a process used to lower the concentration of the original solution by adding solvents. To dilute a solution means to add more solvent without the addition of more solute. Learn how to dilute and concentrate solutions. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Suppose that you. Dilution Of Water By Solute.
From www.slideshare.net
Water and solutions Dilution Of Water By Solute Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. \text{ml}\) of a \(2.0 \: Suppose that you have \(100. Dilution is a process used to lower the concentration of the original solution by adding solvents. You dilute the solution by adding enough water to. Dilution. Dilution Of Water By Solute.
From slideplayer.com
One of the most important substances on Earth. ppt download Dilution Of Water By Solute The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Of course, the resulting solution is thoroughly mixed so as to. The concentrated solution is known. For example, we might say that a glass. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent.. Dilution Of Water By Solute.
From letstalkscience.ca
Osmosis and Its Role in Human Biology and Health Let's Talk Science Dilution Of Water By Solute To dilute a solution means to add more solvent without the addition of more solute. \text{ml}\) of a \(2.0 \: Dilution is the addition of. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. The concentrated solution is known. Dilution is the process of “lowering the concentration of a solute in a. Dilution Of Water By Solute.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Presentation ID643607 Dilution Of Water By Solute Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. \text{ml}\) of a \(2.0 \: The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. To. Dilution Of Water By Solute.
From slideplayer.com
Dilutions. ppt download Dilution Of Water By Solute You dilute the solution by adding enough water to. Dilution is a process used to lower the concentration of the original solution by adding solvents. Learn how to dilute and concentrate solutions. \text{ml}\) of a \(2.0 \: Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Of course, the resulting solution is. Dilution Of Water By Solute.
From www.slideshare.net
Water & solution SCIENCE FORM 2 Dilution Of Water By Solute To dilute a solution means to add more solvent without the addition of more solute. The concentrated solution is known. Of course, the resulting solution is thoroughly mixed so as to. Dilution is the addition of. \text{ml}\) of a \(2.0 \: For example, we might say that a glass. Dilution is the process whereby the concentration of a solution is. Dilution Of Water By Solute.
From saylordotorg.github.io
Solution Concentrations Dilution Of Water By Solute To dilute a solution means to add more solvent without the addition of more solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Of course, the. Dilution Of Water By Solute.
From slideplayer.com
Unit 3, Lesson 14 Dilutions ppt download Dilution Of Water By Solute To dilute a solution means to add more solvent without the addition of more solute. Suppose that you have \(100. \text{ml}\) of a \(2.0 \: Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. You dilute the solution by adding enough water to. Dilution is a process used to lower the concentration. Dilution Of Water By Solute.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Of Water By Solute For example, we might say that a glass. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Learn how to dilute and concentrate solutions. Often, a. Dilution Of Water By Solute.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science State Board, Class 10. Dilution Of Water By Solute The concentrated solution is known. You dilute the solution by adding enough water to. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Of course, the resulting solution is thoroughly mixed so. Dilution Of Water By Solute.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of moles of solute remains Dilution Of Water By Solute Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. To dilute a solution means to add more solvent without the addition of more solute. \text{ml}\) of a \(2.0 \: Of course, the resulting solution. Dilution Of Water By Solute.
From ar.inspiredpencil.com
Dilute Solution Diagram Dilution Of Water By Solute For example, we might say that a glass. \text{ml}\) of a \(2.0 \: Dilution is a process used to lower the concentration of the original solution by adding solvents. Of course, the resulting solution is thoroughly mixed so as to. Suppose that you have \(100. Often, a worker will need to change the concentration of a solution by changing the. Dilution Of Water By Solute.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilution Of Water By Solute For example, we might say that a glass. Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. The dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Often, a worker will need to change the concentration of a solution by changing the amount. Dilution Of Water By Solute.
From slideplayer.com
Mr. Kinton Honors Chemistry ppt download Dilution Of Water By Solute You dilute the solution by adding enough water to. Dilution is the process of “lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Of course, the resulting solution is thoroughly mixed so. Dilution Of Water By Solute.
From slideplayer.com
Part I SOLUTION STOICHIOMETRY ppt download Dilution Of Water By Solute Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution is a process used to lower the concentration of the original solution by adding solvents. Dilution is the process of “lowering the concentration of. Dilution Of Water By Solute.