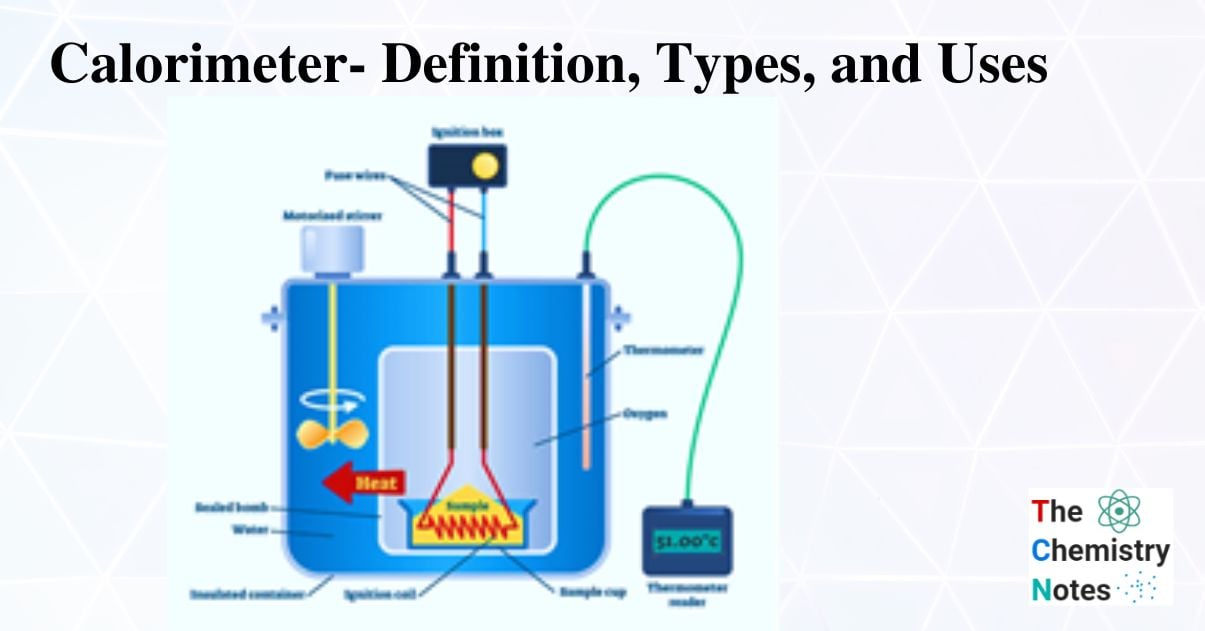
Purpose Of Calorimetry . A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat.
from scienceinfo.com
For example, when an exothermic. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.
Calorimeter Definition, Types and Uses
Purpose Of Calorimetry Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Purpose Of Calorimetry Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. For example, when an exothermic. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. A calorimeter is a device used to measure the amount of heat. Purpose Of Calorimetry.
From quizlet.com
Sketch a simple calorimeter and label the purpose of each co Quizlet Purpose Of Calorimetry A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of. Purpose Of Calorimetry.
From www.slideserve.com
PPT Purpose of the Experiment PowerPoint Presentation, free download ID1994790 Purpose Of Calorimetry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in. Purpose Of Calorimetry.
From hxeoanhdd.blob.core.windows.net
Purpose Of Calibrating A Calorimeter at Debbie Swinson blog Purpose Of Calorimetry Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating. Purpose Of Calorimetry.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of Purpose Of Calorimetry For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Chemists use calorimetry to determine the amount of heat transferred to. Purpose Of Calorimetry.
From www.researchgate.net
Principle of calorimetry. Download Scientific Diagram Purpose Of Calorimetry Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of. Purpose Of Calorimetry.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Purpose Of Calorimetry Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical. Purpose Of Calorimetry.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Engineering Learn Purpose Of Calorimetry For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Purpose Of Calorimetry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1551165 Purpose Of Calorimetry For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Calorimeter, device for measuring the heat. Purpose Of Calorimetry.
From www.animalia-life.club
Calorimeter Diagram Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Purpose Of Calorimetry.
From dvisavzveco.blob.core.windows.net
What Is Calorimetry Thermal Physics at Mary Murphy blog Purpose Of Calorimetry Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Purpose Of Calorimetry.
From quizunderslung.z4.web.core.windows.net
What Is Direct Calorimetry Purpose Of Calorimetry A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Purpose Of Calorimetry.
From users.highland.edu
Calorimetry Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating. Purpose Of Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used for calorimetry, or the process of. Purpose Of Calorimetry.
From www.vrogue.co
Differential Scanning Calorimetry Dsc An Essential An vrogue.co Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. For example, when an exothermic. Chemists use calorimetry to determine the amount of heat transferred to. Purpose Of Calorimetry.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry Purpose Of Calorimetry A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or. Purpose Of Calorimetry.
From www.studocu.com
Lab Manual Calorimetry Cal CALORIMETRY DETERMINATION OF HEATS OF SOLUTION PURPOSE The Purpose Of Calorimetry A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure the amount of. Purpose Of Calorimetry.
From scienceinfo.com
Calorimeter Definition, Types and Uses Purpose Of Calorimetry Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. For. Purpose Of Calorimetry.
From www.youtube.com
Principle of Calorimetry YouTube Purpose Of Calorimetry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions. Purpose Of Calorimetry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Purpose Of Calorimetry Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Purpose Of Calorimetry.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its. Purpose Of Calorimetry.
From saylordotorg.github.io
Calorimetry Purpose Of Calorimetry A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. For example, when an exothermic. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of. Purpose Of Calorimetry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Purpose Of Calorimetry Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical. Purpose Of Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Calorimetry is the process of measuring the amount of heat released. Purpose Of Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID2765956 Purpose Of Calorimetry Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A. Purpose Of Calorimetry.
From hxeoanhdd.blob.core.windows.net
Purpose Of Calibrating A Calorimeter at Debbie Swinson blog Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Purpose Of Calorimetry.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeters Purpose Of Calorimetry A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the amount of heat transferred to or from a system into. Purpose Of Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Purpose Of Calorimetry Chemists use calorimetry to determine the amount of heat transferred to or from a system into its surroundings. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Purpose Of Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Purpose Of Calorimetry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemists use calorimetry to determine the amount of heat transferred to or from a system into its. Purpose Of Calorimetry.
From study.com
Calorimetry Definition, Equation & Types Lesson Purpose Of Calorimetry A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical,. Purpose Of Calorimetry.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.. Purpose Of Calorimetry.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.. Purpose Of Calorimetry.
From www.slideshare.net
Ap chem unit 6 Purpose Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example,. Purpose Of Calorimetry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Purpose Of Calorimetry A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical. Purpose Of Calorimetry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Purpose Of Calorimetry Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat. A calorimeter is a device used to measure the amount of heat involved in a. Purpose Of Calorimetry.