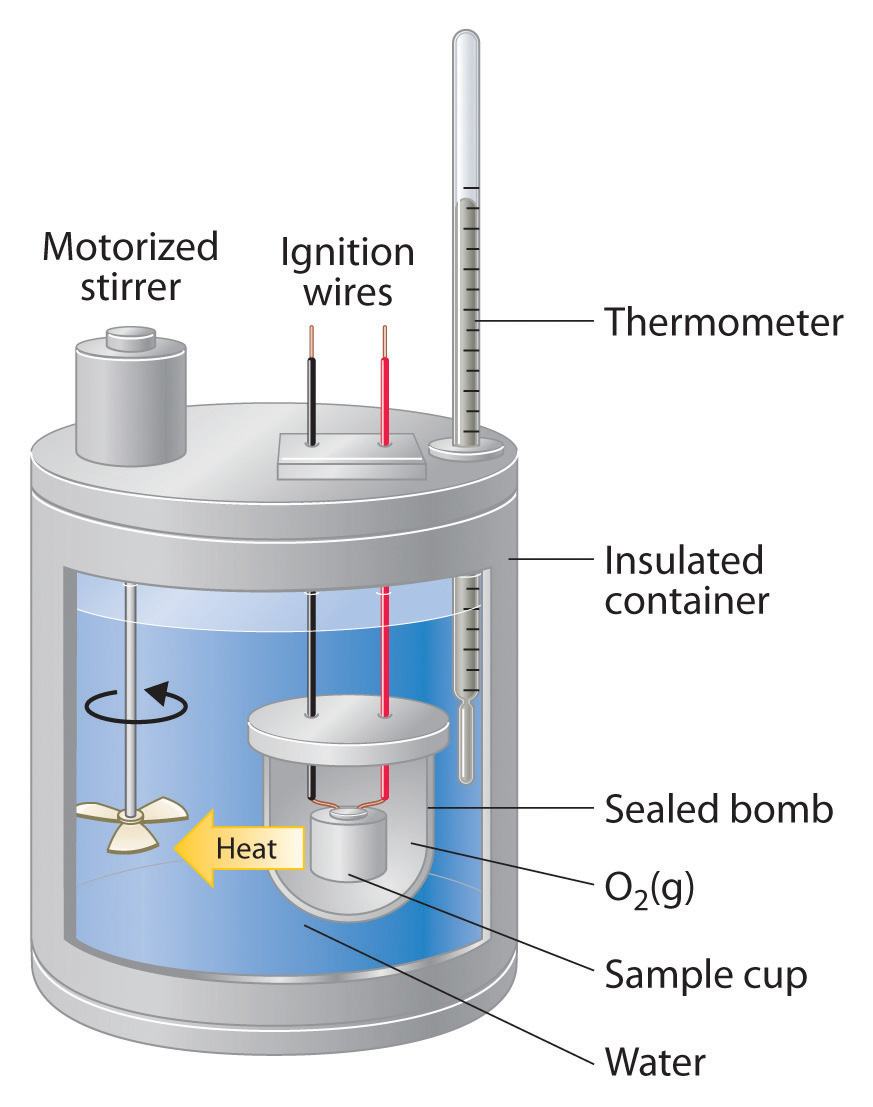
Bomb Calorimeter Key Points . A bucket or container for holding the bomb in a. Four essential parts are required in any bomb calorimeter: A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. The heat released by combustion is. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical reaction. Bomb calorimetry is a technique that allows you to directly measure the heat of combustion of a sample. In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. A bomb or vessel in which the combustible charges can be burned.
from saylordotorg.github.io
Bomb calorimetry is a technique that allows you to directly measure the heat of combustion of a sample. A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. The heat released by combustion is. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. A bucket or container for holding the bomb in a. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical reaction. In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,.
Calorimetry
Bomb Calorimeter Key Points The heat released by combustion is. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. Bomb calorimetry is a technique that allows you to directly measure the heat of combustion of a sample. Four essential parts are required in any bomb calorimeter: A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical reaction. A bucket or container for holding the bomb in a. In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. The heat released by combustion is. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the.
From www.studypool.com
SOLUTION Bomb calorimeter ppt 1 Studypool Bomb Calorimeter Key Points Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. Four essential parts are required in any bomb calorimeter: Bomb calorimetry is a technique that allows you to directly measure. Bomb Calorimeter Key Points.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeters Bomb Calorimeter Key Points Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. In this laboratory, you will. Bomb Calorimeter Key Points.
From pubs.sciepub.com
Figure 1. Diagram of Bomb Calorimeter used in this Study Measuring SizeDependent Enthalpy Bomb Calorimeter Key Points This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. The heat released by combustion is. Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a. In this laboratory, you will determine the standard heat of. Bomb Calorimeter Key Points.
From www.researchgate.net
Schematic sketch of a bomb calorimeter Download Scientific Diagram Bomb Calorimeter Key Points Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. A bucket or container for holding the bomb in a. In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. A bomb or vessel in which the combustible charges can. Bomb Calorimeter Key Points.
From www.shutterstock.com
Diagram Bomb Calorimeter 스톡 벡터(로열티 프리) 1612623736 Shutterstock Bomb Calorimeter Key Points A bucket or container for holding the bomb in a. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. Four essential parts are required in any bomb calorimeter: The heat released. Bomb Calorimeter Key Points.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Bomb Calorimeter Key Points Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a. A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bomb calorimeter is responsible for measuring the amount of heat of. Bomb Calorimeter Key Points.
From ddscalorimeters.com
How does a bomb calorimeter work? Bomb Calorimeter Key Points A bucket or container for holding the bomb in a. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. Four essential parts are required in any bomb calorimeter: Bomb calorimetry is a technique. Bomb Calorimeter Key Points.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID1549939 Bomb Calorimeter Key Points This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. In this laboratory, you will determine the standard heat of formation of a common organic compound such as. Bomb Calorimeter Key Points.
From foodtechnews.in
What Is Bomb Calorimeter🤔 Measurement of Energy Content in food Food Tech NEWS Bomb Calorimeter Key Points Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. Four essential parts are required in any bomb calorimeter: A bomb calorimeter is a device used. Bomb Calorimeter Key Points.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Key Points A bomb or vessel in which the combustible charges can be burned. A bucket or container for holding the bomb in a. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. Four essential parts are required in any bomb calorimeter: A bomb calorimeter is. Bomb Calorimeter Key Points.
From www.slideserve.com
PPT Bomb Calorimeters PowerPoint Presentation, free download ID4499873 Bomb Calorimeter Key Points Bomb calorimetry is a technique that allows you to directly measure the heat of combustion of a sample. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical reaction. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. Four essential. Bomb Calorimeter Key Points.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Key Points Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. Four essential parts are required in any bomb calorimeter: A bomb calorimeter is a scientific instrument designed for measuring the. Bomb Calorimeter Key Points.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Key Points In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. A bomb or vessel in which the combustible charges can be burned. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. A bomb. Bomb Calorimeter Key Points.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Bomb Calorimeter Key Points Bomb calorimetry is a technique that allows you to directly measure the heat of combustion of a sample. In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter is responsible for measuring the amount. Bomb Calorimeter Key Points.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Key Points A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. The heat released by combustion is. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. Bomb calorimetry is a technique that allows you to directly measure the heat of combustion of. Bomb Calorimeter Key Points.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Engineering Learn Bomb Calorimeter Key Points In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. Four essential parts are required in any bomb calorimeter: A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change. Bomb Calorimeter Key Points.
From www.shemmassianconsulting.com
Thermochemistry for the MCAT Everything You Need to Know — Shemmassian Academic Consulting Bomb Calorimeter Key Points A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical reaction. Four essential parts are required in any bomb calorimeter: The heat released by combustion is. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the. Bomb Calorimeter Key Points.
From www.scribd.com
Bomb Calorimeter Principle,formula procedure.docx Calorimetry Enthalpy Bomb Calorimeter Key Points A bomb or vessel in which the combustible charges can be burned. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical reaction. A bomb. Bomb Calorimeter Key Points.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Key Points This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. A bomb or vessel in which the combustible charges can be burned. The heat released by combustion is. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced. Bomb Calorimeter Key Points.
From www.youtube.com
CAL3KAP Oxygen Bomb Calorimeter System Unboxing and Installation YouTube Bomb Calorimeter Key Points Four essential parts are required in any bomb calorimeter: This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. Bomb calorimetry is a technique that allows you to directly measure the heat of combustion of a sample. A bomb calorimeter is responsible for measuring the. Bomb Calorimeter Key Points.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Key Points This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. A bucket or container for holding the bomb in a. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bomb calorimeter is a device used to. Bomb Calorimeter Key Points.
From www.youtube.com
Bomb Calorimeter & Tricks to solve Bomb Calorimeter questions easily YouTube Bomb Calorimeter Key Points This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. The heat released by combustion is. Four essential parts are required in any bomb calorimeter: A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical. Bomb Calorimeter Key Points.
From www.studypool.com
SOLUTION Bomb calorimeter study material Studypool Bomb Calorimeter Key Points A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. A bomb or vessel in which the combustible charges can be burned. Bomb calorimetry is a technique that allows you to directly measure the heat of combustion of a sample. Dulong’s formula used to calculate the theoretical calorific value of fuel. Bomb Calorimeter Key Points.
From www.slideserve.com
PPT Bomb Calorimeter PowerPoint Presentation, free download ID3787850 Bomb Calorimeter Key Points A bomb or vessel in which the combustible charges can be burned. The heat released by combustion is. Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. A bomb calorimeter is. Bomb Calorimeter Key Points.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Key Points This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. A bomb or vessel in which the combustible charges can be burned. In this laboratory, you. Bomb Calorimeter Key Points.
From saylordotorg.github.io
Calorimetry Bomb Calorimeter Key Points The heat released by combustion is. This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced. Bomb Calorimeter Key Points.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Key Points This is the reason which keeps the reaction enthalpy, heats involved in formation, heats involved in the reaction, and change in enthalpy throughout the reaction. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical reaction. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion. Bomb Calorimeter Key Points.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Review for Exams YouTube Bomb Calorimeter Key Points A bucket or container for holding the bomb in a. A bomb or vessel in which the combustible charges can be burned. In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available. Bomb Calorimeter Key Points.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Key Points A bucket or container for holding the bomb in a. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. The heat released by combustion is. Dulong’s formula used to calculate the theoretical calorific. Bomb Calorimeter Key Points.
From www.youtube.com
MECHANICAL ENGINEERING THE BOMB CALORIMETER IS AN APPARATUS TO MEASURE THE YouTube Bomb Calorimeter Key Points A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. The heat released by combustion is. Bomb calorimetry is a technique that allows you to directly measure the heat of combustion of a sample. A bomb or vessel in which the combustible charges can be burned. Dulong’s formula used to calculate. Bomb Calorimeter Key Points.
From byjus.com
What is bomb calorimeter? Bomb Calorimeter Key Points A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. The heat released by combustion is. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is. Bomb Calorimeter Key Points.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co Bomb Calorimeter Key Points The heat released by combustion is. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bucket or container for holding the bomb in a. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. Bomb calorimetry is a technique that allows you. Bomb Calorimeter Key Points.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Bomb Calorimeter Key Points The heat released by combustion is. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. Four essential parts are required in any bomb calorimeter: In this laboratory, you will determine the standard heat of formation of a common organic compound such as naphthalene, glucose, sucrose,. Bomb calorimetry is a technique. Bomb Calorimeter Key Points.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID9276632 Bomb Calorimeter Key Points A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. Dulong’s formula used to calculate the theoretical calorific value of fuel if the ultimate analysis is available and the. A bomb calorimeter is responsible for measuring the amount of heat of combustion which is usually produced in a chemical reaction. Four. Bomb Calorimeter Key Points.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Key Points A bucket or container for holding the bomb in a. A bomb calorimeter is a scientific instrument designed for measuring the heat of combustion of a substance. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of. Four essential parts are required in any bomb calorimeter: In this laboratory, you will. Bomb Calorimeter Key Points.