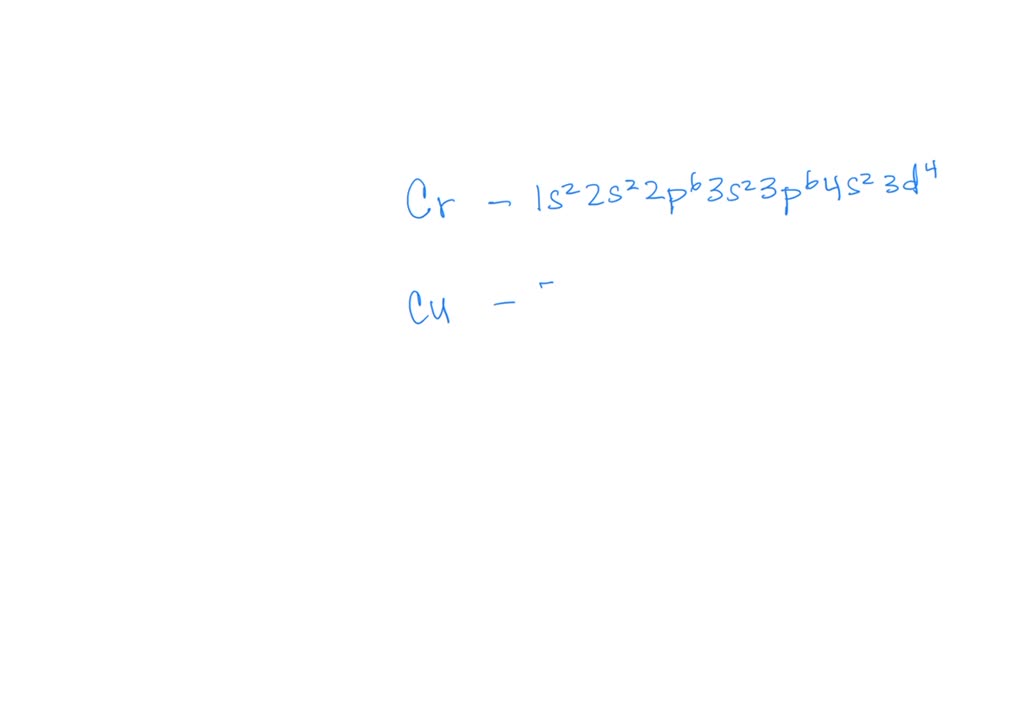Electron Configuration Copper And Chromium . Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Chromium and copper have the following electron configurations, which are different to what you may expect: In chromium, its electron configuration is [ar] 3d^5 4s^1,. How to write the electron configuration for chromium (cr, cr2+, and cr3+) in order to write the chromium electron configuration we first need. The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s.
from www.numerade.com
The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. In chromium, its electron configuration is [ar] 3d^5 4s^1,. Chromium and copper have the following electron configurations, which are different to what you may expect: How to write the electron configuration for chromium (cr, cr2+, and cr3+) in order to write the chromium electron configuration we first need. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals.
SOLVED Using SPECTROSCOPIC notation write the complete electron configuration for the chromium
Electron Configuration Copper And Chromium Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. Chromium and copper have the following electron configurations, which are different to what you may expect: Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. How to write the electron configuration for chromium (cr, cr2+, and cr3+) in order to write the chromium electron configuration we first need. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. In chromium, its electron configuration is [ar] 3d^5 4s^1,. The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect.
From worksheet-z.netlify.app
Electron Configuration Of Copper And Chromium Electron Configuration Copper And Chromium For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know. Electron Configuration Copper And Chromium.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Electron Configuration Copper And Chromium How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. The electron configurations of chromium and copper affect their chemical. Electron Configuration Copper And Chromium.
From slideplayer.com
Electrons in Atoms Electron Configuration ppt download Electron Configuration Copper And Chromium How to write the electron configuration for chromium (cr, cr2+, and cr3+) in order to write the chromium electron configuration we first need. Chromium and copper have the following electron configurations, which are different to what you may expect: For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. The. Electron Configuration Copper And Chromium.
From www.youtube.com
Electronic Configuration of Chromium and Copper Structure of Atom Chemistry Class 11 YouTube Electron Configuration Copper And Chromium Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. In chromium, its electron configuration is [ar] 3d^5 4s^1,. The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. For example, the electron configurations of the transition metals chromium (cr) and. Electron Configuration Copper And Chromium.
From www.youtube.com
How to write/find/do the electron configuration of Cr(Chromium) and Cu(Copper)Exceptions to Electron Configuration Copper And Chromium How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d. Electron Configuration Copper And Chromium.
From askfilo.com
In the above electronic configurations table, the electronic configuratio.. Electron Configuration Copper And Chromium The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. Chromium and copper have the following electron configurations, which are different to what you may expect: In chromium, its electron configuration is [ar] 3d^5 4s^1,. Most of the transition metals have two electrons in the 4s subshell but chromium and. Electron Configuration Copper And Chromium.
From www.sliderbase.com
The d Block Presentation Chemistry Electron Configuration Copper And Chromium Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. In chromium, its electron configuration is [ar] 3d^5 4s^1,. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are. Electron Configuration Copper And Chromium.
From www.youtube.com
ELECTRONIC CONFIGURATION OF COPPER ATOM and STABILITY YouTube Electron Configuration Copper And Chromium Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. In. Electron Configuration Copper And Chromium.
From www.sliderbase.com
The d Block Presentation Chemistry Electron Configuration Copper And Chromium Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. Chromium and copper have the following electron configurations, which are different to what you may expect: For example, the electron configurations of the transition metals chromium (cr) and. Electron Configuration Copper And Chromium.
From www.toppr.com
Write the electronic configuration of chromium and copper Electron Configuration Copper And Chromium Chromium and copper have the following electron configurations, which are different to what you may expect: Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. For example, the electron configurations of. Electron Configuration Copper And Chromium.
From slideplayer.com
Basic Chemistry Chapter 5 Electronic Structure and Periodic Trends ppt download Electron Configuration Copper And Chromium Chromium and copper have the following electron configurations, which are different to what you may expect: Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. In chromium, its electron configuration is [ar] 3d^5 4s^1,. For example, the electron configurations of the transition metals chromium (cr). Electron Configuration Copper And Chromium.
From www.numerade.com
Why do copper and chromium atoms have "unexpected" electron configurations? Numerade Electron Configuration Copper And Chromium Chromium and copper have the following electron configurations, which are different to what you may expect: For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell.. Electron Configuration Copper And Chromium.
From www.slideserve.com
PPT Models of atoms PowerPoint Presentation ID5417370 Electron Configuration Copper And Chromium How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. In chromium, its electron configuration is [ar] 3d^5 4s^1,. Cr is [ar] 3d 5. Electron Configuration Copper And Chromium.
From periodictable.me
Chromium Electron Configuration (Cr) with Orbital Diagram Electron Configuration Copper And Chromium The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. Chromium and copper have the following electron configurations, which are different to what you may expect: Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those. Electron Configuration Copper And Chromium.
From www.youtube.com
Why Copper and Chromium are exceptions? Electronic configuration tricks Afbau Principle Electron Configuration Copper And Chromium In chromium, its electron configuration is [ar] 3d^5 4s^1,. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. How to write the electron configuration for. Electron Configuration Copper And Chromium.
From www.youtube.com
Electronic configuration of chromium and copper simplify electronic configuration with tricks Electron Configuration Copper And Chromium How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. The electron configurations of most transition metals are straightforward, but chromium and copper present. Electron Configuration Copper And Chromium.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Table of Elements and Chemistry Electron Configuration Copper And Chromium How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d. Electron Configuration Copper And Chromium.
From www.youtube.com
Electron Configuration Exceptions to Aufbau's Principle Configuration of Cr, Cu, Mo, and Ag Electron Configuration Copper And Chromium How to write the electron configuration for chromium (cr, cr2+, and cr3+) in order to write the chromium electron configuration we first need. Chromium and copper have the following electron configurations, which are different to what you may expect: Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in. Electron Configuration Copper And Chromium.
From www.youtube.com
Electronic configuration of chromium and copper called exceptions aufbau electronic Electron Configuration Copper And Chromium Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. In chromium, its electron configuration is [ar] 3d^5 4s^1,. The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. Chromium and copper have. Electron Configuration Copper And Chromium.
From www.slideshare.net
Chapter 6 Lecture Electrons in Atoms Electron Configuration Copper And Chromium The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would. Electron Configuration Copper And Chromium.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Electron Configuration Copper And Chromium For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. Chromium and copper have the following electron configurations, which are different to what you may expect:. Electron Configuration Copper And Chromium.
From www.youtube.com
Electron Configuration of Chromium YouTube Electron Configuration Copper And Chromium The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. Chromium. Electron Configuration Copper And Chromium.
From www.pinterest.com
Pin on 100 Steps to SAT II Chemistry Electron Configuration Copper And Chromium For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. Chromium and copper have the following electron configurations, which are different to what you may expect: How to write the. Electron Configuration Copper And Chromium.
From www.youtube.com
Chromium & Copper Electron Configurations part 2 YouTube Electron Configuration Copper And Chromium For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. The electron configurations of. Electron Configuration Copper And Chromium.
From animalia-life.club
Chromium Orbital Diagram Electron Configuration Copper And Chromium Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write. Electron Configuration Copper And Chromium.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Electron Configuration Copper And Chromium Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. The electron configurations of chromium and copper affect their chemical properties due to the presence of. Electron Configuration Copper And Chromium.
From chemaddicts.blogspot.com
Chemaddicts The interpreting electronic structure in box notation Electron Configuration Copper And Chromium The electron configurations of most transition metals are straightforward, but chromium and copper present intriguing. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. Chromium. Electron Configuration Copper And Chromium.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Electron Configuration Copper And Chromium Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. In chromium, its electron configuration is [ar] 3d^5 4s^1,. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. Chromium and copper have the following electron configurations, which are different to what you may expect: How to write. Electron Configuration Copper And Chromium.
From www.pinterest.com.mx
Chromium Art Print by Carlos Clarivan Atom, Electron configuration, Copper art Electron Configuration Copper And Chromium Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. How to write the electron configuration for chromium (cr, cr2+, and cr3+) in order to write the chromium electron configuration we first need. How to write the electron. Electron Configuration Copper And Chromium.
From www.vectorstock.com
Symbol and electron diagram for chromium Vector Image Electron Configuration Copper And Chromium How to write the electron configuration for chromium (cr, cr2+, and cr3+) in order to write the chromium electron configuration we first need. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron configurations of most transition metals are straightforward, but chromium. Electron Configuration Copper And Chromium.
From www.youtube.com
Electron Configuration Exceptions Copper (Cu) and Chromium (Cr) YouTube Electron Configuration Copper And Chromium Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. Chromium and copper have the following electron configurations, which are different to what you may expect: Most of the transition metals have two electrons in the 4s subshell. Electron Configuration Copper And Chromium.
From www.numerade.com
SOLVED Using SPECTROSCOPIC notation write the complete electron configuration for the chromium Electron Configuration Copper And Chromium How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Chromium and copper have the following electron configurations, which are different to what you may expect: Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. How to write the electron configuration. Electron Configuration Copper And Chromium.
From worksheet-z.netlify.app
Electron Configuration Of Copper And Chromium Electron Configuration Copper And Chromium Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. The electron configurations of chromium and copper affect their chemical properties due to the presence of partially filled d orbitals. Most of the transition metals have two electrons in the 4s subshell but chromium and copper have a single electron in their 4s subshell. How to write the. Electron Configuration Copper And Chromium.
From www.youtube.com
Exceptions to Aufbau principle Chromium and Copper configuration BSc physics IIT JAM NEET Electron Configuration Copper And Chromium In chromium, its electron configuration is [ar] 3d^5 4s^1,. For example, the electron configurations of the transition metals chromium (cr) and copper (cu), are not those we would expect. How to write the electron configuration for chromium (cr, cr2+, and cr3+) in order to write the chromium electron configuration we first need. How to write the electron configuration for copper. Electron Configuration Copper And Chromium.
From www.youtube.com
Electron Configuration Exceptions, Chromium and Copper Revison for ALevel Chemistry YouTube Electron Configuration Copper And Chromium How to write the electron configuration for chromium (cr, cr2+, and cr3+) in order to write the chromium electron configuration we first need. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. For example, the electron configurations of the transition metals chromium (cr). Electron Configuration Copper And Chromium.