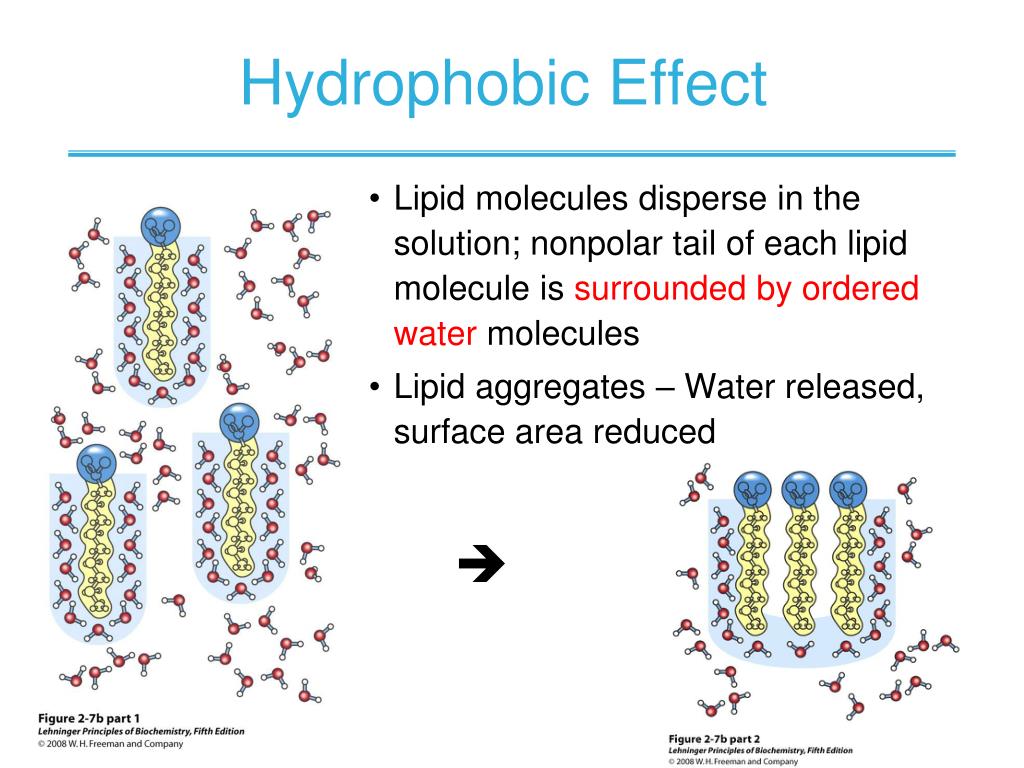
Proteins Are Hydrophobic . They are instead bound to either face of the membrane by noncovalent interactions with. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; We have developed a new computational method that provides the collective response of water to the topological features of. Hydrophobic interactions are important for the folding of proteins. This is important in keeping a protein stable and biologically active, because it allow to the protein. During protein folding, the direct interactions between residues become.
from www.slideserve.com
Hydrophobic interactions are important for the folding of proteins. They are instead bound to either face of the membrane by noncovalent interactions with. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. This is important in keeping a protein stable and biologically active, because it allow to the protein. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; We have developed a new computational method that provides the collective response of water to the topological features of. During protein folding, the direct interactions between residues become.
PPT CHAPTER 2 Water and Aqueous Solutions PowerPoint Presentation, free download ID1343110
Proteins Are Hydrophobic We have developed a new computational method that provides the collective response of water to the topological features of. They are instead bound to either face of the membrane by noncovalent interactions with. We have developed a new computational method that provides the collective response of water to the topological features of. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; Hydrophobic interactions are important for the folding of proteins. During protein folding, the direct interactions between residues become. This is important in keeping a protein stable and biologically active, because it allow to the protein.
From www.slideserve.com
PPT Molecular Cell Biology PowerPoint Presentation ID5351050 Proteins Are Hydrophobic We have developed a new computational method that provides the collective response of water to the topological features of. This is important in keeping a protein stable and biologically active, because it allow to the protein. Hydrophobic interactions are important for the folding of proteins. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in. Proteins Are Hydrophobic.
From en.ppt-online.org
Protein Structure and Function online presentation Proteins Are Hydrophobic They are instead bound to either face of the membrane by noncovalent interactions with. Hydrophobic interactions are important for the folding of proteins. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. Some membrane proteins do not extend into the hydrophobic interior of the lipid. Proteins Are Hydrophobic.
From slcc.pressbooks.pub
7.3 Protein Structure College Biology I Proteins Are Hydrophobic This is important in keeping a protein stable and biologically active, because it allow to the protein. They are instead bound to either face of the membrane by noncovalent interactions with. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. We have developed a new. Proteins Are Hydrophobic.
From courses.lumenlearning.com
Components and Structure OpenStax Biology 2e Proteins Are Hydrophobic During protein folding, the direct interactions between residues become. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. This is important in keeping a protein stable and biologically active,. Proteins Are Hydrophobic.
From www.researchgate.net
LigPlot (left) and hydrophobic protein surface representation (right)... Download Scientific Proteins Are Hydrophobic Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; Hydrophobic interactions are important for the folding of proteins. They are instead bound to either face of the membrane by noncovalent interactions with. We have developed a new computational method that provides the collective response of water to the topological features of. This is. Proteins Are Hydrophobic.
From chem.libretexts.org
Hydrophobic Interactions Chemistry LibreTexts Proteins Are Hydrophobic They are instead bound to either face of the membrane by noncovalent interactions with. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. We have developed a new computational method that provides the collective response of water to the topological features of. This is important. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Chapter Four The ThreeDimensional Structure of Proteins PowerPoint Presentation ID6076424 Proteins Are Hydrophobic During protein folding, the direct interactions between residues become. This is important in keeping a protein stable and biologically active, because it allow to the protein. They are instead bound to either face of the membrane by noncovalent interactions with. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; Hydrophobic interactions are important. Proteins Are Hydrophobic.
From childhealthpolicy.vumc.org
🏆 Hydrophobic and hydrophilic interactions in proteins. How do hydrophobic amino acids and Proteins Are Hydrophobic Hydrophobic interactions are important for the folding of proteins. This is important in keeping a protein stable and biologically active, because it allow to the protein. They are instead bound to either face of the membrane by noncovalent interactions with. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein,. Proteins Are Hydrophobic.
From stock.adobe.com
Phospholipid or phosphatides lipids microscopical structure outline diagram. Labeled educational Proteins Are Hydrophobic Hydrophobic interactions are important for the folding of proteins. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. This is important in keeping a protein stable and biologically active,. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Protein structure PowerPoint Presentation, free download ID9193673 Proteins Are Hydrophobic When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. They are instead bound to either face of the membrane by noncovalent interactions with. This is important in keeping a protein stable and biologically active, because it allow to the protein. Some membrane proteins do not. Proteins Are Hydrophobic.
From plantlet.org
Protein and Peptides An Inevitable Source of Nutrition (Part 2) Plantlet Proteins Are Hydrophobic Hydrophobic interactions are important for the folding of proteins. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. During protein folding, the direct interactions between residues become. They are instead bound to either face of the membrane by noncovalent interactions with. This is important in. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Chapter Four The ThreeDimensional Structure of Proteins PowerPoint Presentation ID6076424 Proteins Are Hydrophobic When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. We have developed a new computational method that provides the collective response of water to the topological features of. They are instead bound to either face of the membrane by noncovalent interactions with. Some membrane proteins. Proteins Are Hydrophobic.
From www.sliderbase.com
Plasma MembraneGateway to the Cell Presentation Biology Proteins Are Hydrophobic They are instead bound to either face of the membrane by noncovalent interactions with. We have developed a new computational method that provides the collective response of water to the topological features of. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; This is important in keeping a protein stable and biologically active,. Proteins Are Hydrophobic.
From mydroll.com
How hydrophobicity shapes protein assemblies Proteins Are Hydrophobic Hydrophobic interactions are important for the folding of proteins. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; This is important in keeping a protein stable and biologically active, because it allow to the protein. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of. Proteins Are Hydrophobic.
From www.slideserve.com
PPT College 2 PowerPoint Presentation ID3287971 Proteins Are Hydrophobic During protein folding, the direct interactions between residues become. They are instead bound to either face of the membrane by noncovalent interactions with. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; This is important in keeping a protein stable and biologically active, because it allow to the protein. We have developed a. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6402527 Proteins Are Hydrophobic We have developed a new computational method that provides the collective response of water to the topological features of. During protein folding, the direct interactions between residues become. Hydrophobic interactions are important for the folding of proteins. This is important in keeping a protein stable and biologically active, because it allow to the protein. When protein folding takes place, the. Proteins Are Hydrophobic.
From slidetodoc.com
Chapter 7 Membrane Structure and Function Cell Membrane Proteins Are Hydrophobic They are instead bound to either face of the membrane by noncovalent interactions with. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; During protein folding, the direct interactions between residues become. Hydrophobic interactions are important for the folding of proteins. We have developed a new computational method that provides the collective response. Proteins Are Hydrophobic.
From www.slideserve.com
PPT College 2 PowerPoint Presentation ID3287971 Proteins Are Hydrophobic Hydrophobic interactions are important for the folding of proteins. This is important in keeping a protein stable and biologically active, because it allow to the protein. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. Some membrane proteins do not extend into the hydrophobic interior. Proteins Are Hydrophobic.
From gaswchampion.weebly.com
Properties of hydrophobic amino acids gaswchampion Proteins Are Hydrophobic We have developed a new computational method that provides the collective response of water to the topological features of. During protein folding, the direct interactions between residues become. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the. Proteins Are Hydrophobic.
From chem.libretexts.org
Hydrophobic Interactions Chemistry LibreTexts Proteins Are Hydrophobic This is important in keeping a protein stable and biologically active, because it allow to the protein. They are instead bound to either face of the membrane by noncovalent interactions with. We have developed a new computational method that provides the collective response of water to the topological features of. When protein folding takes place, the hydrophobic r groups of. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Amino Acids and Protein Structure PowerPoint Presentation, free download ID1869110 Proteins Are Hydrophobic Hydrophobic interactions are important for the folding of proteins. They are instead bound to either face of the membrane by noncovalent interactions with. During protein folding, the direct interactions between residues become. We have developed a new computational method that provides the collective response of water to the topological features of. This is important in keeping a protein stable and. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Levels of Protein Structure PowerPoint Presentation, free download ID493530 Proteins Are Hydrophobic Hydrophobic interactions are important for the folding of proteins. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; They are instead bound to either face of the membrane by noncovalent interactions with. During protein folding, the direct interactions between residues become. We have developed a new computational method that provides the collective response. Proteins Are Hydrophobic.
From www.youtube.com
Thermodynamics of Folding & The Hydrophobic Effect YouTube Proteins Are Hydrophobic Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; Hydrophobic interactions are important for the folding of proteins. We have developed a new computational method that provides the collective response of water to the topological features of. During protein folding, the direct interactions between residues become. They are instead bound to either face. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Lecture 6 PowerPoint Presentation, free download ID297385 Proteins Are Hydrophobic We have developed a new computational method that provides the collective response of water to the topological features of. They are instead bound to either face of the membrane by noncovalent interactions with. This is important in keeping a protein stable and biologically active, because it allow to the protein. Some membrane proteins do not extend into the hydrophobic interior. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Structure of Proteins PowerPoint Presentation, free download ID373647 Proteins Are Hydrophobic Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; They are instead bound to either face of the membrane by noncovalent interactions with. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. We have developed a new computational. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Levels of Protein Structure PowerPoint Presentation, free download ID493530 Proteins Are Hydrophobic This is important in keeping a protein stable and biologically active, because it allow to the protein. During protein folding, the direct interactions between residues become. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. Some membrane proteins do not extend into the hydrophobic interior. Proteins Are Hydrophobic.
From www.slideserve.com
PPT Lipids, Membranes & the First Cells PowerPoint Presentation ID390113 Proteins Are Hydrophobic When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; This is important in keeping a protein stable and biologically active, because it allow to the protein. They are instead. Proteins Are Hydrophobic.
From deallalaf.weebly.com
Bulky hydrophobic amino acids deallalaf Proteins Are Hydrophobic During protein folding, the direct interactions between residues become. They are instead bound to either face of the membrane by noncovalent interactions with. This is important in keeping a protein stable and biologically active, because it allow to the protein. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein,. Proteins Are Hydrophobic.
From www.researchgate.net
(a) Hydrophobic surface areas on the βstrands of the protein 1OUR... Download Scientific Diagram Proteins Are Hydrophobic During protein folding, the direct interactions between residues become. Hydrophobic interactions are important for the folding of proteins. We have developed a new computational method that provides the collective response of water to the topological features of. This is important in keeping a protein stable and biologically active, because it allow to the protein. They are instead bound to either. Proteins Are Hydrophobic.
From www.slideshare.net
Membrane Structure and Function Proteins Are Hydrophobic Hydrophobic interactions are important for the folding of proteins. During protein folding, the direct interactions between residues become. We have developed a new computational method that provides the collective response of water to the topological features of. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; They are instead bound to either face. Proteins Are Hydrophobic.
From www.slideserve.com
PPT AMINO ACIDS PowerPoint Presentation, free download ID6875941 Proteins Are Hydrophobic When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. This is important in keeping a protein stable and biologically active, because it allow to the protein. Hydrophobic interactions are important for the folding of proteins. They are instead bound to either face of the membrane. Proteins Are Hydrophobic.
From coachlasem.weebly.com
Where are hydrophobic amino acids located in cell membrane coachlasem Proteins Are Hydrophobic This is important in keeping a protein stable and biologically active, because it allow to the protein. They are instead bound to either face of the membrane by noncovalent interactions with. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. During protein folding, the direct. Proteins Are Hydrophobic.
From www.slideserve.com
PPT CHAPTER 2 Water and Aqueous Solutions PowerPoint Presentation, free download ID1343110 Proteins Are Hydrophobic This is important in keeping a protein stable and biologically active, because it allow to the protein. They are instead bound to either face of the membrane by noncovalent interactions with. Hydrophobic interactions are important for the folding of proteins. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; When protein folding takes. Proteins Are Hydrophobic.
From bio1151b.nicerweb.com
transmembrane.html 07_08TransmembraneProtein.jpg Proteins Are Hydrophobic During protein folding, the direct interactions between residues become. Hydrophobic interactions are important for the folding of proteins. Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; We have developed a new computational method that provides the collective response of water to the topological features of. When protein folding takes place, the hydrophobic. Proteins Are Hydrophobic.
From www.biologyexams4u.com
Biology Exams 4 U Proteins Are Hydrophobic Some membrane proteins do not extend into the hydrophobic interior of the lipid bilayer at all; Hydrophobic interactions are important for the folding of proteins. When protein folding takes place, the hydrophobic r groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic r. This is important in keeping a protein stable and biologically active,. Proteins Are Hydrophobic.