What Is A Buffer Made Of In Chemistry . A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Ions are atoms or molecules that. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is buffer in chemistry? A buffer solution can resist ph change because of an equilibrium between the acid. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
from uen.pressbooks.pub
It is able to neutralize small. Ions are atoms or molecules that. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution can resist ph change because of an equilibrium between the acid. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is buffer in chemistry? A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
Buffers Introductory Chemistry
What Is A Buffer Made Of In Chemistry Ions are atoms or molecules that. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. What is buffer in chemistry? A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution can resist ph change because of an equilibrium between the acid. Ions are atoms or molecules that. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Made Of In Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer solution is a solution where the ph does not change significantly. What Is A Buffer Made Of In Chemistry.
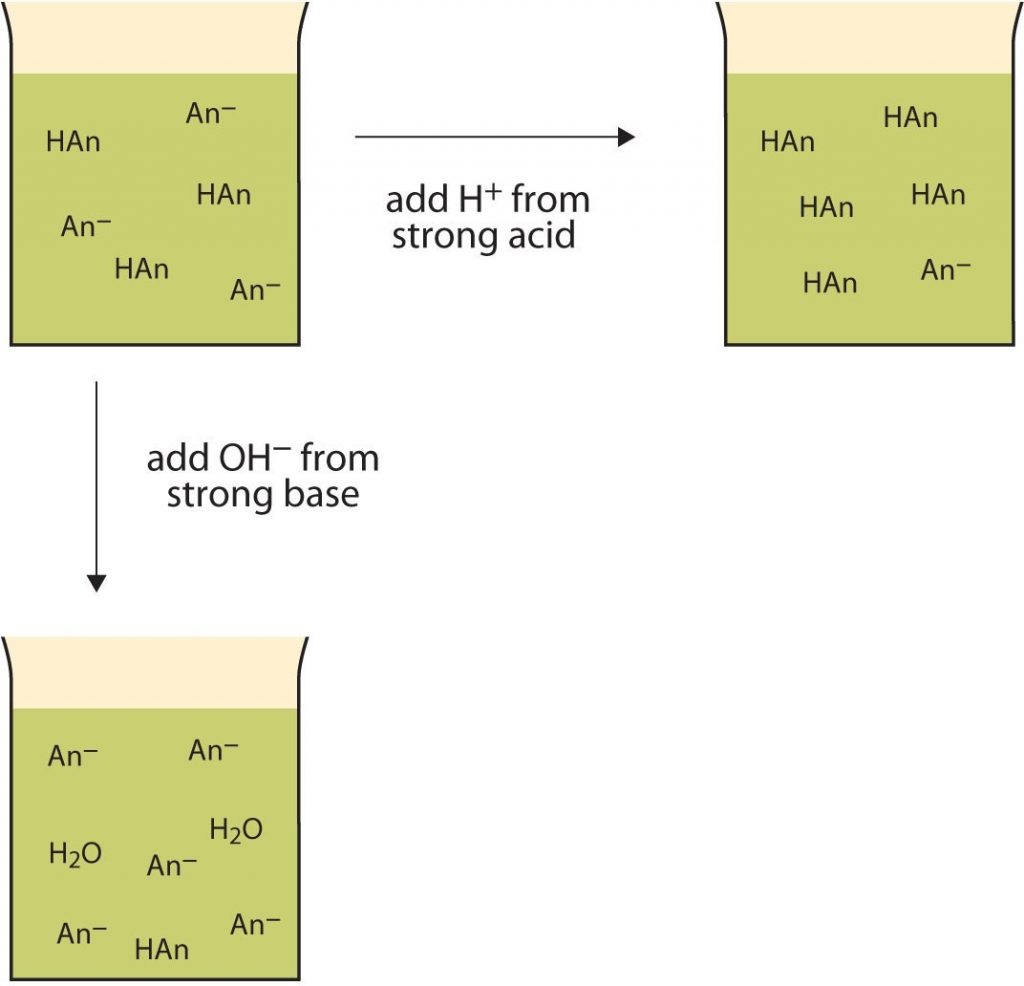
From chem.libretexts.org
10.6 Buffers Chemistry LibreTexts What Is A Buffer Made Of In Chemistry A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Ions are atoms or molecules that. It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. What Is A Buffer Made Of In Chemistry.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID What Is A Buffer Made Of In Chemistry A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Buffer, in chemistry, solution usually containing an acid and a base, or. What Is A Buffer Made Of In Chemistry.
From uen.pressbooks.pub
Buffers Introductory Chemistry What Is A Buffer Made Of In Chemistry Ions are atoms or molecules that. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A buffer solution is a. What Is A Buffer Made Of In Chemistry.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Is A Buffer Made Of In Chemistry Ions are atoms or molecules that. A buffer solution can resist ph change because of an equilibrium between the acid. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or. What Is A Buffer Made Of In Chemistry.
From www.youtube.com
What is a Buffer? (chemistry) YouTube What Is A Buffer Made Of In Chemistry Ions are atoms or molecules that. What is buffer in chemistry? Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature.. What Is A Buffer Made Of In Chemistry.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Made Of In Chemistry What is buffer in chemistry? Ions are atoms or molecules that. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution can resist ph change. What Is A Buffer Made Of In Chemistry.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is A Buffer Made Of In Chemistry Ions are atoms or molecules that. What is buffer in chemistry? Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. It is able to neutralize small.. What Is A Buffer Made Of In Chemistry.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation What Is A Buffer Made Of In Chemistry Ions are atoms or molecules that. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer solution can resist ph change because of an equilibrium between the acid. A buffer is a solution that maintains the stability of a system’s ph level when adding. What Is A Buffer Made Of In Chemistry.
From www.slideshare.net
Buffer system What Is A Buffer Made Of In Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution can resist ph change because of an equilibrium between the acid. Ions. What Is A Buffer Made Of In Chemistry.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Is A Buffer Made Of In Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Ions are atoms or molecules that. A buffer solution can resist. What Is A Buffer Made Of In Chemistry.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog What Is A Buffer Made Of In Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution can resist ph change because of an equilibrium between the acid.. What Is A Buffer Made Of In Chemistry.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Is A Buffer Made Of In Chemistry A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. It is able to neutralize small. What is buffer in chemistry? Buffer, in chemistry, solution usually. What Is A Buffer Made Of In Chemistry.
From www.peoi.org
Chapter 12 Section G Buffers What Is A Buffer Made Of In Chemistry Ions are atoms or molecules that. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution can resist ph change because of an equilibrium between the acid. It is able to neutralize small. A solution whose ph is not altered to any great extent. What Is A Buffer Made Of In Chemistry.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Made Of In Chemistry It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A solution whose ph is not altered to any great extent by the addition of. What Is A Buffer Made Of In Chemistry.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide What Is A Buffer Made Of In Chemistry What is buffer in chemistry? Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution is a solution where the ph does not change. What Is A Buffer Made Of In Chemistry.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is A Buffer Made Of In Chemistry A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Ions are atoms or molecules that. Buffer, in chemistry, solution usually containing an acid and. What Is A Buffer Made Of In Chemistry.
From ceirptus.blob.core.windows.net
What Is Buffer Solution In Chemistry at Elizabeth Olsen blog What Is A Buffer Made Of In Chemistry A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution can resist ph change because of an equilibrium between the acid. A buffer is a solution that maintains. What Is A Buffer Made Of In Chemistry.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Made Of In Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Ions are atoms or molecules that. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. What is buffer in chemistry? It is able to neutralize. What Is A Buffer Made Of In Chemistry.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Is A Buffer Made Of In Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is a solution where the ph does not change significantly on dilution. What Is A Buffer Made Of In Chemistry.
From www.pinterest.co.uk
Buffer Easy Science Chemistry education, Study chemistry, Chemistry What Is A Buffer Made Of In Chemistry A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. Ions are atoms or molecules that. What is buffer in chemistry? Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. It is. What Is A Buffer Made Of In Chemistry.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Buffer Made Of In Chemistry What is buffer in chemistry? A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution can resist ph change because of an equilibrium between the acid. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or. What Is A Buffer Made Of In Chemistry.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks What Is A Buffer Made Of In Chemistry Ions are atoms or molecules that. A buffer solution can resist ph change because of an equilibrium between the acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small. A buffer solution consists of a weak acid and its conjugate base. What Is A Buffer Made Of In Chemistry.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 What Is A Buffer Made Of In Chemistry Ions are atoms or molecules that. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution is a solution where the ph does not. What Is A Buffer Made Of In Chemistry.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is A Buffer Made Of In Chemistry It is able to neutralize small. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution is a solution where the ph does not. What Is A Buffer Made Of In Chemistry.
From treybramirezo.blob.core.windows.net
What Is A Buffer Chemistry at treybramirezo blog What Is A Buffer Made Of In Chemistry A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities. What Is A Buffer Made Of In Chemistry.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses What Is A Buffer Made Of In Chemistry A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer is a solution that can resist ph change upon the addition of an acidic. What Is A Buffer Made Of In Chemistry.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Is A Buffer Made Of In Chemistry Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. What is buffer in chemistry? It is able to neutralize small. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Ions are atoms or. What Is A Buffer Made Of In Chemistry.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Buffer Made Of In Chemistry A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that can resist ph change upon the addition of an. What Is A Buffer Made Of In Chemistry.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is A Buffer Made Of In Chemistry A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer solution can resist ph change because of an equilibrium between the acid. A buffer. What Is A Buffer Made Of In Chemistry.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Is A Buffer Made Of In Chemistry A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a. What Is A Buffer Made Of In Chemistry.
From www.thoughtco.com
What Is a Buffer and How Does It Work? What Is A Buffer Made Of In Chemistry A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Ions are atoms or molecules that. A buffer solution can resist ph change because of an equilibrium between the acid. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or. What Is A Buffer Made Of In Chemistry.
From mydigitalkemistry.com
What is a buffer solution simple definition? Free Chemistry Learning What Is A Buffer Made Of In Chemistry A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. What is buffer in chemistry? A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution is a solution where the ph does not change significantly on dilution or. What Is A Buffer Made Of In Chemistry.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Made Of In Chemistry A buffer solution can resist ph change because of an equilibrium between the acid. It is able to neutralize small. Ions are atoms or molecules that. A solution whose ph is not altered to any great extent by the addition of small quantities of either an acid or base is. A buffer is a solution that maintains the stability of. What Is A Buffer Made Of In Chemistry.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Buffer Made Of In Chemistry A buffer solution can resist ph change because of an equilibrium between the acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. It. What Is A Buffer Made Of In Chemistry.