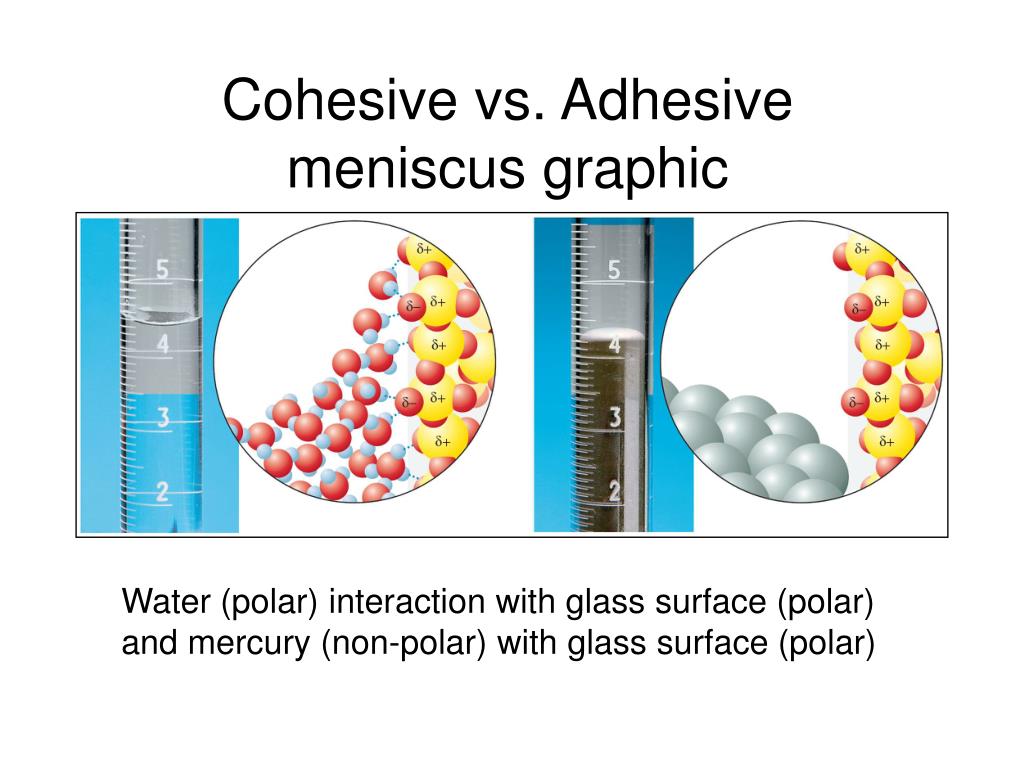
Adhesive Forces Characteristics . Attractive forces between molecules of different types are called adhesive forces. Use the principles of cohesive and adhesive forces to explain this situation. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids. Such forces cause liquid drops to cling to window panes, for example. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. Such forces cause liquid drops to cling to window panes, for example. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. Propose different types of forces that adhesive forces can build on. In this section we examine. Attractive forces between molecules of different types are called adhesive forces. This general effect is called surface tension. Attractive forces between molecules of different types are called adhesive forces. Attractive forces between molecules of different types are called adhesive forces. Explain why a water strider can glide on the water with the knowledge of cohesion in water.
from www.slideserve.com
Use the principles of cohesive and adhesive forces to explain this situation. In this section we examine. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. Explain why a water strider can glide on the water with the knowledge of cohesion in water. This general effect is called surface tension. Propose different types of forces that adhesive forces can build on. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. In this section we examine.
PPT Chpt 10 Condensed Phases PowerPoint Presentation, free download
Adhesive Forces Characteristics In this section we examine. This general effect is called surface tension. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Attractive forces between molecules of different types are called adhesive forces. Explain why a water strider can glide on the water with the knowledge of cohesion in water. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids. Attractive forces between molecules of different types are called adhesive forces. Use the principles of cohesive and adhesive forces to explain this situation. Attractive forces between molecules of different types are called adhesive forces. Propose different types of forces that adhesive forces can build on. Such forces cause liquid drops to cling to window panes, for example. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. The main difference between adhesive and cohesive forces is that adhesive forces involve the attraction between molecules of different substances, whereas cohesive forces involve the attraction between molecules of the same substance. Such forces cause liquid drops to cling to window panes, for example. In this section we examine. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID5852920 Adhesive Forces Characteristics Use the principles of cohesive and adhesive forces to explain this situation. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. Such forces cause liquid drops to cling to window panes, for example. In this section we examine. Attractive forces between molecules of different types are called adhesive forces. Attractive forces between molecules of different. Adhesive Forces Characteristics.
From www.researchgate.net
Adhesion Force Measurement A Between particle and substrate B Between Adhesive Forces Characteristics This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. Attractive forces between molecules of different types are called adhesive forces. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. In this section. Adhesive Forces Characteristics.
From www.iqsdirectory.com
Adhesive Tape What Is It? How Is It Made? Uses, Application Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Such forces cause liquid drops to cling to window panes, for example. Propose different types of forces that adhesive forces can build on. This general effect is called surface tension. In. Adhesive Forces Characteristics.
From www.expii.com
Cohesion and Adhesion — Definition & Overview Expii Adhesive Forces Characteristics Such forces cause liquid drops to cling to window panes, for example. Such forces cause liquid drops to cling to window panes, for example. Such forces cause liquid drops to cling to window panes, for example. Attractive forces between molecules of different types are called adhesive forces. In this section we examine effects directly attributable to cohesive and adhesive forces. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint Adhesive Forces Characteristics Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. This general effect is called surface tension. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops. Adhesive Forces Characteristics.
From www.researchgate.net
Structure of adhesive joint and effect of surface energy. Download Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. Propose different types of forces that adhesive forces can build on. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Attractive forces between molecules of different types are called adhesive forces. Explain why a water strider can glide on the. Adhesive Forces Characteristics.
From www.youtube.com
Adhesion, Cohesion forces YouTube Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Explain why a water strider can glide on the water with the knowledge of cohesion in water. In this section we examine. Propose different types of forces that adhesive forces can. Adhesive Forces Characteristics.
From www.researchgate.net
Mechanical characteristics of adhesives Download Scientific Diagram Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. This general effect is called surface tension. Propose different types of forces that adhesive forces can build on. Explain why a water strider can glide on the water with the knowledge of cohesion in water.. Adhesive Forces Characteristics.
From chem.libretexts.org
Cohesive and Adhesive Forces Chemistry LibreTexts Adhesive Forces Characteristics Explain why a water strider can glide on the water with the knowledge of cohesion in water. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. Such forces cause liquid drops to. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Review Intermolecular Forces PowerPoint Presentation, free Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. Such forces cause liquid drops to cling to window panes, for example. Such forces cause liquid drops to cling to window panes, for example. Molecules on the surface are pulled inward by cohesive forces, reducing the surface. Adhesive Forces Characteristics.
From avopix.com
Illustration of chemical. Cohesive forces are Royalty Free Stock Adhesive Forces Characteristics Explain why a water strider can glide on the water with the knowledge of cohesion in water. Attractive forces between molecules of different types are called adhesive forces. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. This general effect is called surface tension. Such forces cause liquid drops to cling to window panes, for. Adhesive Forces Characteristics.
From www.dreamstime.com
Cohesive Forces and Adhesive Forces Diagram Stock Illustration Adhesive Forces Characteristics Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. Attractive forces between molecules of different types are called adhesive forces. Use the principles of cohesive and adhesive forces to explain this situation. Such forces cause liquid drops to cling to window panes, for example. In this section we examine. Cohesive forces between molecules cause the. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Liquids and Intermolecular Forces PowerPoint Presentation, free Adhesive Forces Characteristics This general effect is called surface tension. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. In this section we examine. Propose different types of forces that adhesive forces can build on. In this section we examine. Attractive forces between molecules of different types are called. Adhesive Forces Characteristics.
From www.learncram.com
Adhesive Force in Physics Meaning, Example Surface Tension Learn Cram Adhesive Forces Characteristics Explain why a water strider can glide on the water with the knowledge of cohesion in water. Propose different types of forces that adhesive forces can build on. In this section we examine. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. The main difference between. Adhesive Forces Characteristics.
From www.pinterest.com
Difference Between Cohesion and Adhesion Definition, Relationship Adhesive Forces Characteristics This general effect is called surface tension. Attractive forces between molecules of different types are called adhesive forces. In this section we examine. Attractive forces between molecules of different types are called adhesive forces. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids. The main difference between adhesive and cohesive forces is that adhesive. Adhesive Forces Characteristics.
From www.youtube.com
Intermolecular Forces In Fluids Cohesive And Adhesive Forces Fluid Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. Propose different types of forces that adhesive forces can build on. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids. Use the principles of cohesive and adhesive forces to explain this. Adhesive Forces Characteristics.
From dianakruwyates.blogspot.com
Describe the Difference Between Adhesion and Cohesion DianakruwYates Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. Propose different types of forces that adhesive forces can build on. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids. Attractive forces between molecules of different types are called adhesive forces.. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID27506 Adhesive Forces Characteristics Such forces cause liquid drops to cling to window panes, for example. Propose different types of forces that adhesive forces can build on. Use the principles of cohesive and adhesive forces to explain this situation. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. In this section we examine. Such forces cause liquid drops to. Adhesive Forces Characteristics.
From www.researchgate.net
Schematic illustration of the internal structure of an adhesive polymer Adhesive Forces Characteristics Use the principles of cohesive and adhesive forces to explain this situation. In this section we examine. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called surface tension. Attractive forces between. Adhesive Forces Characteristics.
From www.vrogue.co
11 Biology Cohesive And Adhesive Forces In Water Comp vrogue.co Adhesive Forces Characteristics Such forces cause liquid drops to cling to window panes, for example. Attractive forces between molecules of different types are called adhesive forces. In this section we examine. Such forces cause liquid drops to cling to window panes, for example. Use the principles of cohesive and adhesive forces to explain this situation. In this section we examine effects directly attributable. Adhesive Forces Characteristics.
From www.researchgate.net
The adhesive force characteristics comparing particles from rural and Adhesive Forces Characteristics Propose different types of forces that adhesive forces can build on. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. In this section we examine. Such forces cause liquid drops to cling to window panes, for example. Such forces cause liquid drops to cling to window panes, for example. Such forces cause liquid drops to. Adhesive Forces Characteristics.
From www.researchgate.net
The adhesive force curve of (a) the SUB surface and (b) the LIS Adhesive Forces Characteristics Use the principles of cohesive and adhesive forces to explain this situation. In this section we examine. Propose different types of forces that adhesive forces can build on. Such forces cause liquid drops to cling to window panes, for example. Attractive forces between molecules of different types are called adhesive forces. Molecules on the surface are pulled inward by cohesive. Adhesive Forces Characteristics.
From phyvlab.bjtu.edu.cn
Viscosity, Cohesive and Adhesive Forces, Surface … · F Adhesive Forces Characteristics This general effect is called surface tension. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. In this section we examine. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. Such forces. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Chpt 10 Condensed Phases PowerPoint Presentation, free download Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. Propose different types of forces that adhesive forces can build on. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. In this section we examine. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. In. Adhesive Forces Characteristics.
From www.researchgate.net
Schematic view of the representative adhesive bonding interface; (A Adhesive Forces Characteristics Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. Such forces cause liquid drops to cling to window panes, for example. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. Such forces cause liquid drops to cling to window panes, for. Adhesive Forces Characteristics.
From www.youtube.com
COHESIVE AND ADHESIVE FORCES understand in simple way YouTube Adhesive Forces Characteristics Such forces cause liquid drops to cling to window panes, for example. Attractive forces between molecules of different types are called adhesive forces. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids. Such forces cause liquid drops to cling to window panes, for example. In this section we examine. Attractive forces between molecules of. Adhesive Forces Characteristics.
From www.celadon.com.tw
Intruction of Adhesive Tape The Science of Adhesive Tapes Celadon Adhesive Forces Characteristics Such forces cause liquid drops to cling to window panes, for example. In this section we examine. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. Attractive forces between molecules of different types are called adhesive forces. Attractive forces between molecules of different types are called. Adhesive Forces Characteristics.
From www.youtube.com
Cohesive and Adhesive Forces YouTube Adhesive Forces Characteristics In this section we examine. Attractive forces between molecules of different types are called adhesive forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Explain why a water strider can glide on the water with the knowledge of cohesion in water. Molecules on the surface are pulled inward by cohesive. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Ch 11 States of Matter and Intermolecular Forces PowerPoint Adhesive Forces Characteristics This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. Attractive forces between molecules of different types are called adhesive forces. Attractive forces between molecules of different types are called adhesive forces. Use the principles of cohesive and adhesive forces to explain this situation. Attractive forces between. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Capillary Action PowerPoint Presentation, free download ID2833294 Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. Explain why a water strider can glide on the water with the. Adhesive Forces Characteristics.
From www.youtube.com
Cohesion and adhesion class 8 physics matter cohesive force Adhesive Forces Characteristics Such forces cause liquid drops to cling to window panes, for example. Such forces cause liquid drops to cling to window panes, for example. Explain why a water strider can glide on the water with the knowledge of cohesion in water. Attractive forces between molecules of different types are called adhesive forces. In this section we examine. In this section. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Properties of Liquids and Solids PowerPoint Presentation, free Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak van der waals forces,. Propose different types of forces that adhesive forces can build on. Such forces cause liquid drops to cling to window panes, for example. Attractive forces between. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID Adhesive Forces Characteristics Such forces cause liquid drops to cling to window panes, for example. Such forces cause liquid drops to cling to window panes, for example. Explain why a water strider can glide on the water with the knowledge of cohesion in water. This chapter focuses on the description of the main forces responsible for adhesion, from strong covalent bonds to weak. Adhesive Forces Characteristics.
From www.walker-rubber.co.uk
How do you make rubber adhesive? Walker Rubber Adhesive Forces Characteristics Attractive forces between molecules of different types are called adhesive forces. In this section we examine. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. Explain why a water strider can glide on the water with the knowledge of cohesion in water. Cohesive forces between molecules cause the surface of a liquid to contract to. Adhesive Forces Characteristics.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID5852920 Adhesive Forces Characteristics This general effect is called surface tension. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids. In this section we examine. Propose different types of forces that adhesive forces can build. Adhesive Forces Characteristics.