Zinc Experiment . The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Zn + i 2 → zni 2 Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and. The purpose of this experiment is to determine the enthalpy change for the displacement reaction: Teacher only uses knife to slice up lemons. Cbse class 9 chemistry practicals and experiments on experiment on the reaction of zinc with dilute sulphuric acid with detailed instructions, safety advice and background information. A brilliant flash of light, followed by hot sparks, a hissing sound and a mushroom. In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. It is no more difficult than that! As the reaction progresses, you can observe the formation of bubbles as the hydrogen gas is released. Class tests the lemon experiment for zinc. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Zinc powder is added to a solution of iodine in ethanol. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction.
from www.chegg.com
Zinc powder is added to a solution of iodine in ethanol. It is no more difficult than that! In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. Class sets up the zinc plating experiment (exp 4) and. Class tests the lemon experiment for zinc. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and. The purpose of this experiment is to determine the enthalpy change for the displacement reaction: This reaction is highly exothermic, meaning it releases a significant amount of heat.
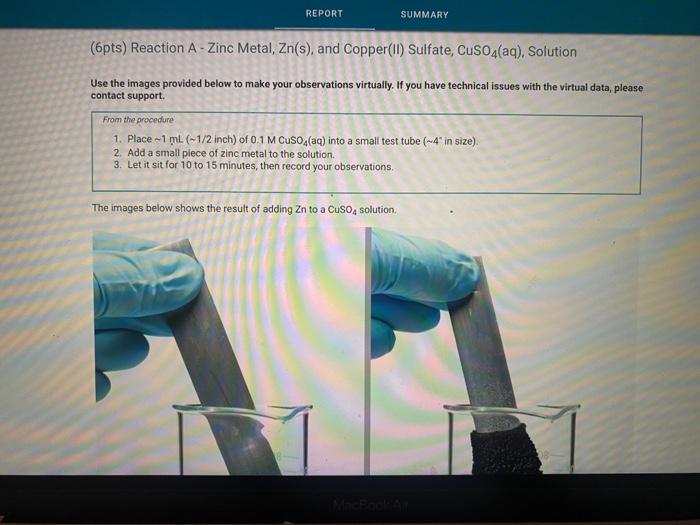
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II)
Zinc Experiment Teacher only uses knife to slice up lemons. Zinc powder is added to a solution of iodine in ethanol. Class sets up the zinc plating experiment (exp 4) and. Cbse class 9 chemistry practicals and experiments on experiment on the reaction of zinc with dilute sulphuric acid with detailed instructions, safety advice and background information. Class tests the lemon experiment for zinc. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Teacher only uses knife to slice up lemons. Zn + i 2 → zni 2 The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. As the reaction progresses, you can observe the formation of bubbles as the hydrogen gas is released. In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and. A brilliant flash of light, followed by hot sparks, a hissing sound and a mushroom.
From www.alamy.com
Daniell element galvanic cell with zinc and copper Stock Photo Zinc Experiment The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. It is no more difficult than that! Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. Class sets up the zinc plating experiment (exp 4) and. The purpose of this. Zinc Experiment.
From www.youtube.com
The action of heat on zinc II nitrate Laboratory experiments on salts Zinc Experiment Class tests the lemon experiment for zinc. A brilliant flash of light, followed by hot sparks, a hissing sound and a mushroom. Cbse class 9 chemistry practicals and experiments on experiment on the reaction of zinc with dilute sulphuric acid with detailed instructions, safety advice and background information. The experiment reinforces ideas about energy changes during reactions, the reactivity series. Zinc Experiment.
From bsbgroup.com
Corrosion protection of structural steel Brewer Smith Brewer Group Zinc Experiment Class tests the lemon experiment for zinc. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc. Zinc Experiment.
From www.youtube.com
Experiment Chemie Erhitzen von Zink und Schwefel YouTube Zinc Experiment The purpose of this experiment is to determine the enthalpy change for the displacement reaction: Zn + i 2 → zni 2 By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and. Class tests the lemon experiment for zinc. The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals. Zinc Experiment.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc Experiment An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. Zn + i 2 → zni 2 Class tests the lemon experiment for zinc. The zinc begins to dissolve in the acid, producing hydrogen gas and. Zinc Experiment.
From www.studypool.com
SOLUTION Exp 4 Zinc experiment Studypool Zinc Experiment This reaction is highly exothermic, meaning it releases a significant amount of heat. It is no more difficult than that! Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. Class sets up the zinc plating experiment (exp 4) and. Class tests the lemon experiment for zinc. A brilliant flash of light,. Zinc Experiment.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Zinc Experiment Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Zinc powder is added to a solution of iodine in ethanol. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and. The. Zinc Experiment.
From www.youtube.com
DemonstrationsExperiment ZinkPulver in wässriger Kupfersulfatlösung Zinc Experiment Class tests the lemon experiment for zinc. As the reaction progresses, you can observe the formation of bubbles as the hydrogen gas is released. In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. It is no more. Zinc Experiment.
From www.youtube.com
Chemische Reaktion Zink Schwefel Zinksulfid mit Experiment Zinc Experiment The purpose of this experiment is to determine the enthalpy change for the displacement reaction: Class tests the lemon experiment for zinc. In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. This reaction is highly exothermic,. Zinc Experiment.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Zinc Experiment As the reaction progresses, you can observe the formation of bubbles as the hydrogen gas is released. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Teacher only uses knife to slice up lemons. Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. In. Zinc Experiment.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Experiment In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. A brilliant flash of light, followed by hot sparks, a hissing sound and a mushroom. The purpose of this experiment is to determine the enthalpy change for the displacement reaction: Cbse class 9 chemistry practicals and experiments on experiment on the reaction of zinc. Zinc Experiment.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Zinc Experiment A brilliant flash of light, followed by hot sparks, a hissing sound and a mushroom. Class tests the lemon experiment for zinc. Class sets up the zinc plating experiment (exp 4) and. The purpose of this experiment is to determine the enthalpy change for the displacement reaction: An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by. Zinc Experiment.
From 2012books.lardbucket.org
Electrochemistry Zinc Experiment It is no more difficult than that! Cbse class 9 chemistry practicals and experiments on experiment on the reaction of zinc with dilute sulphuric acid with detailed instructions, safety advice and background information. Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. The experiment reinforces ideas about energy changes during reactions,. Zinc Experiment.
From www.doubtnut.com
When dilute hydrochloric acid is added to granulated zinc placed i Zinc Experiment A brilliant flash of light, followed by hot sparks, a hissing sound and a mushroom. Class sets up the zinc plating experiment (exp 4) and. Teacher only uses knife to slice up lemons. Zn + i 2 → zni 2 The purpose of this experiment is to determine the enthalpy change for the displacement reaction: By adding an excess of. Zinc Experiment.
From question.pandai.org
Rusting Zinc Experiment By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and. Teacher only uses knife to slice up lemons. The purpose of this experiment is to determine the enthalpy change for the displacement reaction: Zinc powder is added to a solution of iodine in ethanol. Zn + i 2 → zni 2 It is no. Zinc Experiment.
From www.youtube.com
Reaction of Zinc with HCl 10th class YouTube Zinc Experiment This reaction is highly exothermic, meaning it releases a significant amount of heat. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Cbse class 9 chemistry practicals and experiments on experiment on the reaction of zinc with dilute sulphuric acid with detailed instructions, safety advice and background information. As the reaction progresses,. Zinc Experiment.
From www.sciencelearn.org.nz
Zinc in sphalerite — Science Learning Hub Zinc Experiment In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained. Zinc Experiment.
From www.mdpi.com
Molecules Free FullText Green Synthesis of Zinc Oxide Zinc Experiment By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. This reaction is highly exothermic, meaning it releases a significant amount of heat. Class sets up the zinc plating experiment (exp 4) and. The experiment reinforces ideas about energy. Zinc Experiment.
From www.experimente.physik.uni-freiburg.de
Photoeffekt mit Zinkplatte — Experimente Physikalisches Institut Zinc Experiment Teacher only uses knife to slice up lemons. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. As the reaction progresses, you can observe the. Zinc Experiment.
From www.youtube.com
Reaction of Zinc Granules with Dilute Sulphuric Acid and Testing Zinc Experiment Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. It is no more difficult than that! The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. As the reaction progresses,. Zinc Experiment.
From studylib.net
Zinc Coating Experiment Zinc Experiment This reaction is highly exothermic, meaning it releases a significant amount of heat. It is no more difficult than that! As the reaction progresses, you can observe the formation of bubbles as the hydrogen gas is released. Teacher only uses knife to slice up lemons. Zn + i 2 → zni 2 Class tests the lemon experiment for zinc. By. Zinc Experiment.
From schneidersci.blogspot.com
Schneider Science Zinc & Hydrochloric Acid Zinc Experiment An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. It is no more difficult than that! The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for. Zinc Experiment.
From www.youtube.com
Make Zinc Powder by Electrolysis YouTube Zinc Experiment Class tests the lemon experiment for zinc. Zinc powder is added to a solution of iodine in ethanol. This reaction is highly exothermic, meaning it releases a significant amount of heat. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. A brilliant flash of light, followed by hot sparks, a hissing sound and. Zinc Experiment.
From www.youtube.com
Zinc + Vinegar Reaction Experiment YouTube Zinc Experiment Class tests the lemon experiment for zinc. Zn + i 2 → zni 2 It is no more difficult than that! The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Class sets up the zinc plating experiment. Zinc Experiment.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Zinc Experiment In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Class tests the lemon experiment for zinc. As the reaction progresses, you can observe the formation of. Zinc Experiment.
From www.youtube.com
Zink, Schwefel & 🔥 YouTube Zinc Experiment Class sets up the zinc plating experiment (exp 4) and. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Class tests the lemon experiment for zinc. Teacher only uses knife to slice up lemons. This reaction is highly exothermic, meaning it releases a significant amount of heat. The experiment reinforces ideas about energy. Zinc Experiment.
From www.youtube.com
Chemistry Experiment How To Make Zinc Acetate Full HD video YouTube Zinc Experiment It is no more difficult than that! A brilliant flash of light, followed by hot sparks, a hissing sound and a mushroom. Teacher only uses knife to slice up lemons. Class sets up the zinc plating experiment (exp 4) and. Cbse class 9 chemistry practicals and experiments on experiment on the reaction of zinc with dilute sulphuric acid with detailed. Zinc Experiment.
From brainly.in
9.1 a The diagram shows an experiment where zinc metal is added to Zinc Experiment Zn + i 2 → zni 2 Cbse class 9 chemistry practicals and experiments on experiment on the reaction of zinc with dilute sulphuric acid with detailed instructions, safety advice and background information. Class tests the lemon experiment for zinc. The purpose of this experiment is to determine the enthalpy change for the displacement reaction: In this demonstration a mixture. Zinc Experiment.
From www.alamy.com
Reactivity of metals. Magnesium, zinc, iron and lead in dilute Stock Zinc Experiment An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. Zinc powder is added to a solution of iodine in ethanol. Zn + i 2 → zni 2 A brilliant flash of light, followed by hot sparks, a. Zinc Experiment.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Experiment The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Teacher only uses knife to slice up lemons. Class sets up the zinc plating experiment (exp 4) and. Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. Zinc powder is added to a solution of iodine. Zinc Experiment.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Experiment The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. As the reaction progresses, you can observe the formation of bubbles as. Zinc Experiment.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Zinc Experiment The experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. The purpose of this experiment is to. Zinc Experiment.
From schneidersci.blogspot.com
Schneider Science Zinc & Hydrochloric Acid Zinc Experiment The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. A brilliant flash of light, followed by hot sparks, a hissing sound and a mushroom. It is no more difficult than that! Zinc powder is added to a solution of iodine in ethanol. Chemistry practical class 9 covers the list of practicals, experiments and activities. Zinc Experiment.
From www.youtube.com
zink + sulphuric acid practical zinc and sulphuric acid experiment Zinc Experiment A brilliant flash of light, followed by hot sparks, a hissing sound and a mushroom. Enthalpy change is simply a measure of the amount of heat evolved or absorbed during a reaction. Teacher only uses knife to slice up lemons. In this demonstration a mixture of zinc and sulfur produces an unusual chemical reaction when heated. This reaction is highly. Zinc Experiment.
From www.youtube.com
Reaction Of Zinc Granules With Dilute Sulphuric Acid And Testing Zinc Experiment Class tests the lemon experiment for zinc. It is no more difficult than that! This reaction is highly exothermic, meaning it releases a significant amount of heat. Chemistry practical class 9 covers the list of practicals, experiments and activities to be performed for the exam. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution,. Zinc Experiment.