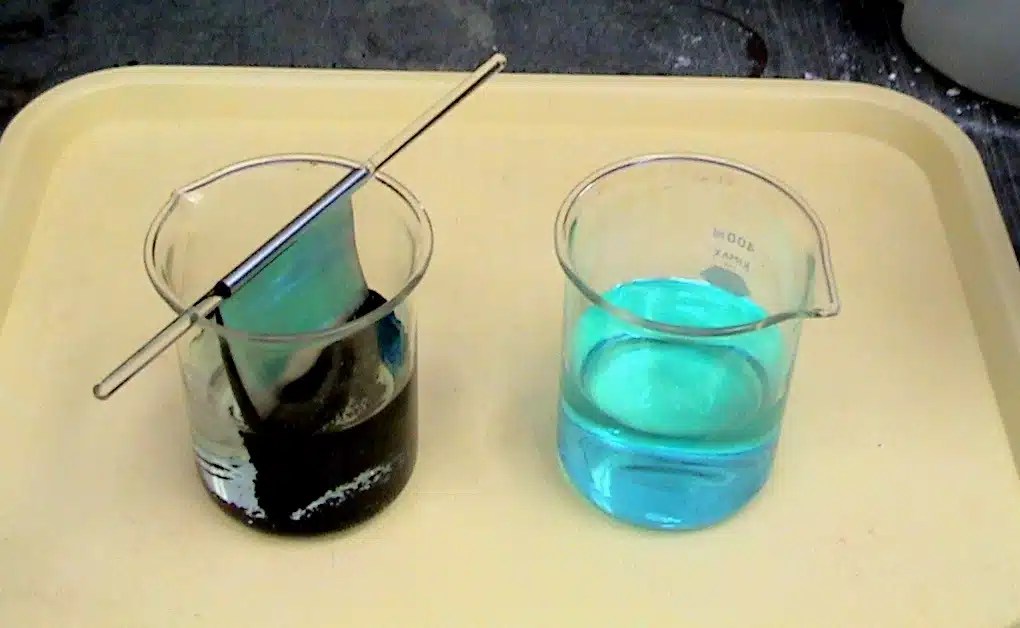
Copper Sulfate And Zinc . Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc (zn) is more reactive than copper (cu), therefore it can. What happens when you add zinc to a solution of copper sulfate? When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Then a zinc electrode is placed in the zinc sulfate solution. A piece of zinc is placed in a copper sulfate solution. Identifying the half reactions to see what got oxidized and reduced. What happens when zinc reacts with copper sulphate solution? Connecting the copper electrode to the zinc electrode. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. A zinc sulfate solution is floated on top of the copper sulfate solution; 0.5 or 1m copper sulfate;
from schoolworkhelper.net
Then a zinc electrode is placed in the zinc sulfate solution. What happens when you add zinc to a solution of copper sulfate? When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Identifying the half reactions to see what got oxidized and reduced. A zinc sulfate solution is floated on top of the copper sulfate solution; Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. 0.5 or 1m copper sulfate; What happens when zinc reacts with copper sulphate solution? A piece of zinc is placed in a copper sulfate solution.
Single Displacement Reactions Lab Explained SchoolWorkHelper
Copper Sulfate And Zinc What happens when zinc reacts with copper sulphate solution? What happens when you add zinc to a solution of copper sulfate? If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Then a zinc electrode is placed in the zinc sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; Connecting the copper electrode to the zinc electrode. 0.5 or 1m copper sulfate; What happens when zinc reacts with copper sulphate solution? Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. A piece of zinc is placed in a copper sulfate solution. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Zinc (zn) is more reactive than copper (cu), therefore it can. Identifying the half reactions to see what got oxidized and reduced.
From www.dreamstime.com
Copper Sulfate Molecular Structure Stock Photos Free & RoyaltyFree Copper Sulfate And Zinc When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Connecting the copper electrode to the zinc electrode. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions.. Copper Sulfate And Zinc.
From uwaterloo.ca
Zinc metal in a solution of copper(II) sulfate and sulfuric acid from Copper Sulfate And Zinc If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. What happens when zinc reacts with copper sulphate solution? Then a zinc electrode is placed in the zinc sulfate solution. 0.5 or 1m copper sulfate; Connecting the copper electrode to the zinc electrode. Identifying the half reactions to. Copper Sulfate And Zinc.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Copper Sulfate And Zinc Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. Zinc (zn) is more reactive than copper (cu), therefore it can. A piece of zinc is placed in a copper sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; It is. Copper Sulfate And Zinc.
From eng.hekserij.nl
Copper (II) sulfate (T) Hekserij Hekserij Copper Sulfate And Zinc A zinc sulfate solution is floated on top of the copper sulfate solution; A piece of zinc is placed in a copper sulfate solution. Identifying the half reactions to see what got oxidized and reduced. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. Connecting the. Copper Sulfate And Zinc.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0148 Science Copper Sulfate And Zinc Then a zinc electrode is placed in the zinc sulfate solution. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to. Copper Sulfate And Zinc.
From fineartamerica.com
Zinc Reacting With Copper Sulfate Photograph by GIPhotoStock Copper Sulfate And Zinc 0.5 or 1m copper sulfate; If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. A zinc sulfate solution is floated on top of the copper sulfate solution; What happens when zinc reacts with copper sulphate solution? Zinc (zn) is more reactive than copper (cu), therefore it can.. Copper Sulfate And Zinc.
From www.youtube.com
Make a Copper sulfate and zinc battery "Gravity" design YouTube Copper Sulfate And Zinc When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. Connecting the copper electrode to the zinc electrode.. Copper Sulfate And Zinc.
From www.pastpapersinside.com
Redox Reaction Past Papers Inside Copper Sulfate And Zinc A piece of zinc is placed in a copper sulfate solution. Connecting the copper electrode to the zinc electrode. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a. Copper Sulfate And Zinc.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Copper Sulfate And Zinc 0.5 or 1m copper sulfate; If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Then a zinc electrode is placed in the zinc sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; What happens when zinc reacts with copper sulphate solution?. Copper Sulfate And Zinc.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Copper Sulfate And Zinc 0.5 or 1m copper sulfate; Zinc (zn) is more reactive than copper (cu), therefore it can. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. What happens when zinc reacts with copper sulphate solution? Identifying the half reactions to see what got oxidized and reduced. Then. Copper Sulfate And Zinc.
From uhighlsu.web.fc2.com
copper sulfate and zinc Copper Sulfate And Zinc What happens when you add zinc to a solution of copper sulfate? Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. Identifying the half reactions to see what got oxidized and reduced. It is commonly known that when zinc metal is placed in a solution of. Copper Sulfate And Zinc.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry Copper Sulfate And Zinc What happens when zinc reacts with copper sulphate solution? Connecting the copper electrode to the zinc electrode. Identifying the half reactions to see what got oxidized and reduced. Then a zinc electrode is placed in the zinc sulfate solution. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs,. Copper Sulfate And Zinc.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Copper Sulfate And Zinc What happens when you add zinc to a solution of copper sulfate? If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. A piece of zinc is placed in a copper sulfate solution. It is commonly known that when zinc metal is placed in a solution of copper. Copper Sulfate And Zinc.
From www.indiamart.com
Copper Sulphate Crystal, 50 Kg, Grade Standard Industrial Grade, Rs Copper Sulfate And Zinc Connecting the copper electrode to the zinc electrode. A piece of zinc is placed in a copper sulfate solution. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. What happens when zinc reacts with copper sulphate solution? Identifying the half reactions. Copper Sulfate And Zinc.
From www.youtube.com
Reaction of Copper Sulphate (CuSO4) with Sodium Hydroxide (NaOH) YouTube Copper Sulfate And Zinc Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. What happens when zinc reacts with copper sulphate solution? Identifying the half reactions to see what got oxidized and reduced. 0.5 or 1m copper sulfate; It is commonly known that when zinc metal is placed in a. Copper Sulfate And Zinc.
From readingandwritingprojectcom.web.fc2.com
formula for copper sulfate Copper Sulfate And Zinc Zinc (zn) is more reactive than copper (cu), therefore it can. A zinc sulfate solution is floated on top of the copper sulfate solution; A piece of zinc is placed in a copper sulfate solution. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is. Copper Sulfate And Zinc.
From www.sciencephoto.com
Copper Sulphate Stock Image C028/1274 Science Photo Library Copper Sulfate And Zinc If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. A zinc sulfate solution is floated on top of the copper sulfate solution; Connecting the copper electrode to the zinc electrode. 0.5 or 1m copper sulfate; When a strip of zinc metal is placed into a blue solution. Copper Sulfate And Zinc.
From www.indiamart.com
Copper Sulphate Powder, Crystal at Rs 200/kg in Ahmedabad ID 25575336091 Copper Sulfate And Zinc Connecting the copper electrode to the zinc electrode. What happens when you add zinc to a solution of copper sulfate? It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. A zinc sulfate solution is floated on top of the copper sulfate. Copper Sulfate And Zinc.
From www.youtube.com
Zinc and copper sulfate react Investigating Chemical Reactions Copper Sulfate And Zinc Connecting the copper electrode to the zinc electrode. Zinc (zn) is more reactive than copper (cu), therefore it can. What happens when you add zinc to a solution of copper sulfate? It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. If. Copper Sulfate And Zinc.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Copper Sulfate And Zinc 0.5 or 1m copper sulfate; Then a zinc electrode is placed in the zinc sulfate solution. It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. A piece of zinc is placed in a copper sulfate solution. What happens when you add. Copper Sulfate And Zinc.
From www.youtube.com
Copper Sulfate + Magnesium YouTube Copper Sulfate And Zinc If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. 0.5 or 1m copper sulfate; Then a zinc electrode is placed in the zinc sulfate solution. Identifying the half reactions to see what got oxidized and reduced. Zinc (zn) is more reactive than copper (cu), therefore it can.. Copper Sulfate And Zinc.
From www.chegg.com
Solved 08 00 (4pts) For the reaction between zinc and Copper Sulfate And Zinc 0.5 or 1m copper sulfate; Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. What happens when zinc reacts with copper sulphate solution? When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as. Copper Sulfate And Zinc.
From www.alamy.com
Copper(II) sulfate and Zinc Powder in test tube with plug cap. Cosmetic Copper Sulfate And Zinc It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. What happens when. Copper Sulfate And Zinc.
From www.youtube.com
Zinc and Copper Sulphate reaction (Displacement Reaction) Zn and CuSO4 Copper Sulfate And Zinc A piece of zinc is placed in a copper sulfate solution. What happens when zinc reacts with copper sulphate solution? Connecting the copper electrode to the zinc electrode. Then a zinc electrode is placed in the zinc sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; When a strip of zinc metal is placed. Copper Sulfate And Zinc.
From www.sciencephoto.com
Nickel Reacting With Copper Sulfate Stock Image C028/0162 Science Copper Sulfate And Zinc What happens when you add zinc to a solution of copper sulfate? A zinc sulfate solution is floated on top of the copper sulfate solution; Zinc (zn) is more reactive than copper (cu), therefore it can. What happens when zinc reacts with copper sulphate solution? When a strip of zinc metal is placed into a blue solution of copper (ii). Copper Sulfate And Zinc.
From www.teachoo.com
MCQ (Sample Paper) In reaction of iron with copper sulphate solution Copper Sulfate And Zinc It is commonly known that when zinc metal is placed in a solution of copper (ii) sulfate, a displacement reaction occurs, and elemental copper is deposited onto the. Then a zinc electrode is placed in the zinc sulfate solution. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc. Copper Sulfate And Zinc.
From brainly.in
write the chemical reaction between iron and copper sulphate solution Copper Sulfate And Zinc A zinc sulfate solution is floated on top of the copper sulfate solution; Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. Zinc (zn) is more reactive than copper (cu), therefore it can. 0.5 or 1m copper sulfate; What happens when zinc reacts with copper sulphate. Copper Sulfate And Zinc.
From ferroussulphat.com
Copper Sulfate Pentahydrate Micro/Big Crystal GUANGXI TENGXIAN Copper Sulfate And Zinc When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Then a zinc electrode is placed in the zinc sulfate solution. What happens when zinc reacts with copper sulphate solution? A zinc sulfate solution is floated on top of the copper. Copper Sulfate And Zinc.
From www.canoeracing.org.uk
Copper And Zinc Copper Sulfate And Zinc Then a zinc electrode is placed in the zinc sulfate solution. A piece of zinc is placed in a copper sulfate solution. Identifying the half reactions to see what got oxidized and reduced. What happens when zinc reacts with copper sulphate solution? 0.5 or 1m copper sulfate; If left in the solution for a longer period of time, the zinc. Copper Sulfate And Zinc.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Copper Sulfate And Zinc What happens when zinc reacts with copper sulphate solution? If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Zinc (zn) is more reactive than copper (cu), therefore it can. Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a. Copper Sulfate And Zinc.
From jinchangshengchem.com
Copper Sulphate, Zinc Sulphate, Ammonium Dibutyl Jinchangsheng Copper Sulfate And Zinc A zinc sulfate solution is floated on top of the copper sulfate solution; If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to zinc ions. Identifying the half reactions to see what got oxidized and reduced. 0.5 or 1m copper sulfate; What happens when zinc reacts with copper sulphate solution?. Copper Sulfate And Zinc.
From ceaikxdx.blob.core.windows.net
What Is Electrochemistry Apex at Lauretta Brogdon blog Copper Sulfate And Zinc Zinc (zn) is more reactive than copper (cu), therefore it can. Connecting the copper electrode to the zinc electrode. A piece of zinc is placed in a copper sulfate solution. Identifying the half reactions to see what got oxidized and reduced. What happens when zinc reacts with copper sulphate solution? What happens when you add zinc to a solution of. Copper Sulfate And Zinc.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Copper Sulfate And Zinc Zinc is a more reactive metal than copper, thus it is able to displace copper metal from a solution containing copper (ii) ions. What happens when zinc reacts with copper sulphate solution? Connecting the copper electrode to the zinc electrode. If left in the solution for a longer period of time, the zinc will gradually decay due to oxidation to. Copper Sulfate And Zinc.
From www.youtube.com
Copper Sulfate + Zinc YouTube Copper Sulfate And Zinc Then a zinc electrode is placed in the zinc sulfate solution. What happens when you add zinc to a solution of copper sulfate? What happens when zinc reacts with copper sulphate solution? A piece of zinc is placed in a copper sulfate solution. Zinc (zn) is more reactive than copper (cu), therefore it can. Identifying the half reactions to see. Copper Sulfate And Zinc.
From socratic.org
Can you describe the process that releases electrons in a zinc copper Copper Sulfate And Zinc What happens when zinc reacts with copper sulphate solution? Identifying the half reactions to see what got oxidized and reduced. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zinc is a more reactive metal than copper, thus it is. Copper Sulfate And Zinc.