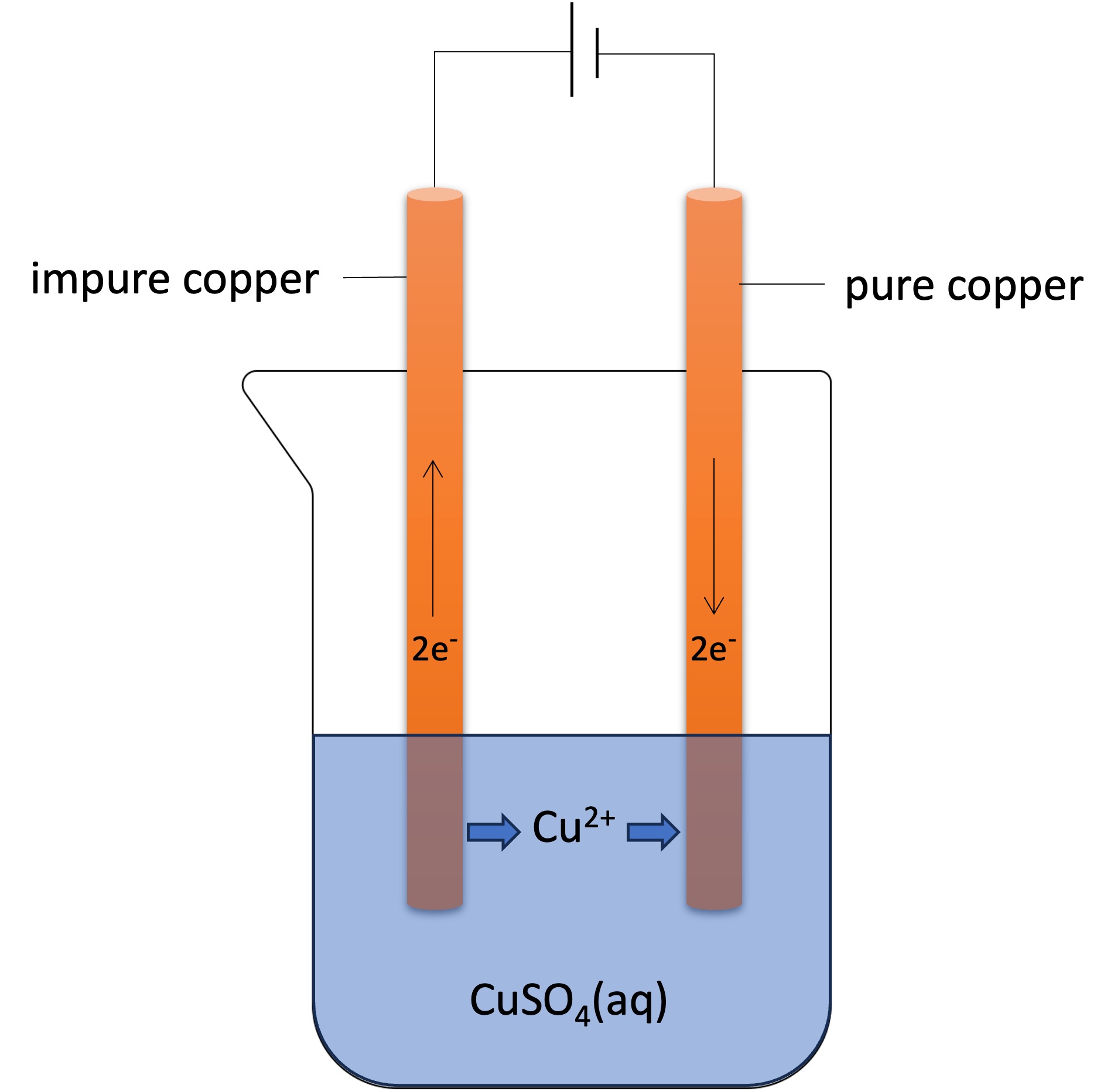
Purification Chemistry Electrolysis . Purification of copper involves using copper electrodes in an electrolyte. Electricity is passed through solutions containing copper compounds, such as copper sulfate. Reactive metals are extracted from their ores. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. Electrolysis can be used to purify metals by separating them from their impurities. Students will appreciate the relevance of these processes. In this process, the anode would be made from impure copper and the. Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. Copper is purified by electrolysis. Copper can be purified by electrolysis. The slideshow shows what happens. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. The electrolyte must contain the same ions, in solution, as the metal. What are electrolytes and what happens in electrolysis?
from chemistry.al-study.com
Students will appreciate the relevance of these processes. The slideshow shows what happens. Reactive metals are extracted from their ores. Copper can be purified by electrolysis. In this process, the anode would be made from impure copper and the. Purification of copper involves using copper electrodes in an electrolyte. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Copper is purified by electrolysis. Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine.
4.1 Electrolysis IGCSE and A Level Chemistry Learning site
Purification Chemistry Electrolysis The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Copper can be purified by electrolysis. Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. Electricity is passed through solutions containing copper compounds, such as copper sulfate. The slideshow shows what happens. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. In this process, the anode would be made from impure copper and the. Copper is purified by electrolysis. What are electrolytes and what happens in electrolysis? The electrolyte must contain the same ions, in solution, as the metal. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. Students will appreciate the relevance of these processes. Reactive metals are extracted from their ores. Electrolysis can be used to purify metals by separating them from their impurities. Purification of copper involves using copper electrodes in an electrolyte.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science Purification Chemistry Electrolysis Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. Copper is purified by electrolysis. Electrolysis can be used to purify metals by separating them from their impurities. In this process, the anode would be made from impure copper and the. Copper can be purified by electrolysis. Purification of copper involves using. Purification Chemistry Electrolysis.
From chem.libretexts.org
17.7 Electrolysis Chemistry LibreTexts Purification Chemistry Electrolysis Purification of copper involves using copper electrodes in an electrolyte. Electricity is passed through solutions containing copper compounds, such as copper sulfate. Electrolysis can be used to purify metals by separating them from their impurities. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. The purification uses an electrolyte of copper(ii). Purification Chemistry Electrolysis.
From mavink.com
Electrolysis Cell Diagram Purification Chemistry Electrolysis What are electrolytes and what happens in electrolysis? Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. Purification of copper involves using copper electrodes in an electrolyte. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. The slideshow shows what happens. The purification uses an electrolyte of. Purification Chemistry Electrolysis.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Purification Chemistry Electrolysis The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. The electrolyte must contain the same ions, in solution, as the metal. Electrolysis can be used to purify metals by separating them from their impurities. Reactive metals are extracted from their ores. Purification of copper involves using copper electrodes. Purification Chemistry Electrolysis.
From chemistrymsq10.blogspot.com
Grade10 CHAPTER 3 ELECTROLYSIS SEMESTER 1 Purification Chemistry Electrolysis During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. What are electrolytes and what happens in electrolysis? Reactive metals are extracted from their ores. The slideshow shows what happens. The purification uses an. Purification Chemistry Electrolysis.
From www.youtube.com
Electroplating & The Purification Of Copper (GCSE Chemistry) YouTube Purification Chemistry Electrolysis In this process, the anode would be made from impure copper and the. The slideshow shows what happens. Electrolysis can be used to purify metals by separating them from their impurities. Reactive metals are extracted from their ores. Copper is purified by electrolysis. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and. Purification Chemistry Electrolysis.
From question.pandai.org
Electrolytic Cell Purification Chemistry Electrolysis In this process, the anode would be made from impure copper and the. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. Electrolysis can be used to purify metals by separating them from their impurities. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper. Purification Chemistry Electrolysis.
From www.dreamstime.com
Copper Purification by Electrolysis, Extracts Pure Copper from Purification Chemistry Electrolysis What are electrolytes and what happens in electrolysis? Purification of copper involves using copper electrodes in an electrolyte. Electrolysis can be used to purify metals by separating them from their impurities. Reactive metals are extracted from their ores. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Electricity is passed through. Purification Chemistry Electrolysis.
From chemistry.analia-sanchez.net
Electrochemistry Notes Chemistry Classes / Ronald Reagan S.H.S. Purification Chemistry Electrolysis In this process, the anode would be made from impure copper and the. Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. Copper is purified by electrolysis. Reactive metals are extracted from their ores. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. The. Purification Chemistry Electrolysis.
From chemistry.analia-sanchez.net
Electrochemistry Notes Chemistry Classes / Ronald Reagan S.H.S. Purification Chemistry Electrolysis Copper is purified by electrolysis. In this process, the anode would be made from impure copper and the. The slideshow shows what happens. The electrolyte must contain the same ions, in solution, as the metal. Reactive metals are extracted from their ores. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine.. Purification Chemistry Electrolysis.
From www.youtube.com
Grade 12 ElectrolysisPurification of Copper ore Exam Question YouTube Purification Chemistry Electrolysis The slideshow shows what happens. Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Reactive metals are extracted from their ores. The electrolyte must contain the same ions, in solution, as the metal. Copper is purified by electrolysis.. Purification Chemistry Electrolysis.
From www.youtube.com
Electrolytic purification, Chemistry Lecture Sabaq.pk YouTube Purification Chemistry Electrolysis The slideshow shows what happens. The electrolyte must contain the same ions, in solution, as the metal. Purification of copper involves using copper electrodes in an electrolyte. Electrolysis can be used to purify metals by separating them from their impurities. Copper is purified by electrolysis. Students will appreciate the relevance of these processes. The purification uses an electrolyte of copper(ii). Purification Chemistry Electrolysis.
From chemistry.al-study.com
4.1 Electrolysis IGCSE and A Level Chemistry Learning site Purification Chemistry Electrolysis In this process, the anode would be made from impure copper and the. Students will appreciate the relevance of these processes. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. The slideshow shows. Purification Chemistry Electrolysis.
From mavink.com
Electrolysis Set Up Diagram Purification Chemistry Electrolysis The electrolyte must contain the same ions, in solution, as the metal. What are electrolytes and what happens in electrolysis? Electricity is passed through solutions containing copper compounds, such as copper sulfate. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. Electrolysis is at the heart of aluminium. Purification Chemistry Electrolysis.
From www.youtube.com
SPM Form 5 Chemistry Chapter 1 Application of Electrolysis in Purification Chemistry Electrolysis Copper can be purified by electrolysis. Purification of copper involves using copper electrodes in an electrolyte. Students will appreciate the relevance of these processes. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium. Purification Chemistry Electrolysis.
From www.youtube.com
Purification of metal through electrolysis YouTube Purification Chemistry Electrolysis Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. Reactive metals are extracted from their ores. The electrolyte must contain the same ions, in solution, as the metal. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. Electrolysis can be used to purify metals. Purification Chemistry Electrolysis.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Purification Chemistry Electrolysis The slideshow shows what happens. Electricity is passed through solutions containing copper compounds, such as copper sulfate. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. Electrolysis can be used to purify metals by separating them from their impurities. The electrolyte must contain the same ions, in solution,. Purification Chemistry Electrolysis.
From www.dreamstime.com
Electrolysis of Copper Sulfate Solution with Impure Copper Anode and Purification Chemistry Electrolysis Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. The slideshow shows what happens. Electricity is passed through solutions containing copper compounds, such as copper sulfate. Reactive metals are extracted from their ores.. Purification Chemistry Electrolysis.
From www.youtube.com
GCSE Chemistry 19 Purifying Copper using Electrolysis YouTube Purification Chemistry Electrolysis The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. In this process, the anode would be made from impure copper and the. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. Electricity is passed through solutions containing copper compounds,. Purification Chemistry Electrolysis.
From www.youtube.com
Using Electrolysis To Extract Metals (GCSE Chemistry) YouTube Purification Chemistry Electrolysis The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. The slideshow shows what happens. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. Reactive metals are extracted from their ores. Copper is purified by electrolysis. In this process, the. Purification Chemistry Electrolysis.
From www.savemyexams.com
Electrolysis of Aqueous Solutions (HL) HL IB Chemistry Revision Notes Purification Chemistry Electrolysis Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. Electrolysis can be used to purify metals by separating them from their impurities. What are electrolytes and what happens in electrolysis? Students will appreciate the relevance of these processes.. Purification Chemistry Electrolysis.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Purification Chemistry Electrolysis Reactive metals are extracted from their ores. Purification of copper involves using copper electrodes in an electrolyte. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Electrolysis can be used to purify metals by separating them from their impurities. Electricity is passed through solutions containing copper compounds, such as copper sulfate.. Purification Chemistry Electrolysis.
From www.youtube.com
Electrolysis Copper Purification YouTube Purification Chemistry Electrolysis During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. The electrolyte must contain the same ions, in solution, as the metal. The slideshow shows what happens. Purification of copper involves using copper electrodes in an electrolyte. Reactive metals are extracted from their ores. Electrolysis is at the heart of aluminium production,. Purification Chemistry Electrolysis.
From saylordotorg.github.io
Electrochemistry Purification Chemistry Electrolysis Electrolysis can be used to purify metals by separating them from their impurities. The slideshow shows what happens. In this process, the anode would be made from impure copper and the. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. During electrolysis, the anode loses mass as copper. Purification Chemistry Electrolysis.
From www.vedantu.com
In the purification of copper, the setup of the electrolytic cell is Purification Chemistry Electrolysis Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. The slideshow shows what happens. What are electrolytes and what happens in electrolysis? Electricity is passed through solutions containing copper compounds, such as copper sulfate. Copper can be purified by electrolysis. Copper is purified by electrolysis. Purification of copper involves using copper. Purification Chemistry Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Purification Chemistry Electrolysis Students will appreciate the relevance of these processes. Electrolysis can be used to purify metals by separating them from their impurities. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Copper can be purified by electrolysis. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium. Purification Chemistry Electrolysis.
From www.teachit.co.uk
Copper electrolysis diagramsKS4 worksheetTeachit Purification Chemistry Electrolysis Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. Purification of copper involves using copper electrodes in an electrolyte. Electricity is passed through solutions containing copper compounds, such as copper sulfate. In this process, the anode would be made from impure copper and the. The purification uses an electrolyte of copper(ii). Purification Chemistry Electrolysis.
From paulaabbmathis.blogspot.com
Anode and Cathode in Electrolysis PaulaabbMathis Purification Chemistry Electrolysis The electrolyte must contain the same ions, in solution, as the metal. The slideshow shows what happens. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Purification of copper involves. Purification Chemistry Electrolysis.
From enagic-asia.com
The electrolysis process Enagic Kangen Water Purification Chemistry Electrolysis Electrolysis can be used to purify metals by separating them from their impurities. What are electrolytes and what happens in electrolysis? In this process, the anode would be made from impure copper and the. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. Purification of copper involves using copper electrodes in. Purification Chemistry Electrolysis.
From byjus.com
In the purification of impure copper using electrolysis, which of the Purification Chemistry Electrolysis Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. Reactive metals are extracted from their ores. Students will appreciate the relevance of these processes. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. Electrolysis can be used to purify metals by separating them from. Purification Chemistry Electrolysis.
From chemistryguru.com.sg
Purification of Copper via Electrolysis Purification Chemistry Electrolysis Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. Electrolysis can be used to purify metals by separating them from their impurities. The slideshow shows what happens. Copper is purified by electrolysis. Reactive metals are extracted from their ores. Electricity is passed through solutions containing copper compounds, such as copper (ii). Purification Chemistry Electrolysis.
From www.reicat.de
Proven purification of hydrogen from PEM electrolysis Englisch Purification Chemistry Electrolysis Copper can be purified by electrolysis. Students will appreciate the relevance of these processes. Electrolysis can be used to purify metals by separating them from their impurities. Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. In this process, the anode would be made from impure copper and the. During electrolysis, the anode loses mass as. Purification Chemistry Electrolysis.
From www.youtube.com
Chemistry Application of Electrolysis (Purification) YouTube Purification Chemistry Electrolysis Reactive metals are extracted from their ores. The slideshow shows what happens. Electrolysis is at the heart of aluminium production, copper purification, and the production of sodium hydroxide and chlorine. Copper can be purified by electrolysis. The electrolyte must contain the same ions, in solution, as the metal. During electrolysis, the anode loses mass as copper dissolves, and the cathode. Purification Chemistry Electrolysis.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Purification Chemistry Electrolysis Purification of copper involves using copper electrodes in an electrolyte. Copper is purified by electrolysis. Students will appreciate the relevance of these processes. The purification uses an electrolyte of copper(ii) sulphate solution, impure copper anodes, and strips of high purity copper for the cathodes. What are electrolytes and what happens in electrolysis? In this process, the anode would be made. Purification Chemistry Electrolysis.
From phys.org
A powerful catalyst for electrolysis of water that could help harness Purification Chemistry Electrolysis Electricity is passed through solutions containing copper compounds, such as copper (ii) sulfate. During electrolysis, the anode loses mass as copper dissolves, and the cathode gains mass as copper is deposited. Electricity is passed through solutions containing copper compounds, such as copper sulfate. Students will appreciate the relevance of these processes. The slideshow shows what happens. The purification uses an. Purification Chemistry Electrolysis.