What Dissolves Calcium Phosphate . When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. In general, ionic substance with anions that are weak bases dissolve better in acidic solution. It is insoluble in ethanol, and acetic. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. The solubility of calcium salts. It is the solubility that determines the direction of many reactions that. What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? Solubility is one of the most important properties of calcium phosphate salts. This is the same problem as above. As the water is made more.
from www.mdpi.com
It is insoluble in ethanol, and acetic. This is the same problem as above. It is the solubility that determines the direction of many reactions that. In general, ionic substance with anions that are weak bases dissolve better in acidic solution. Solubility is one of the most important properties of calcium phosphate salts. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. The solubility of calcium salts. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. As the water is made more. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium.
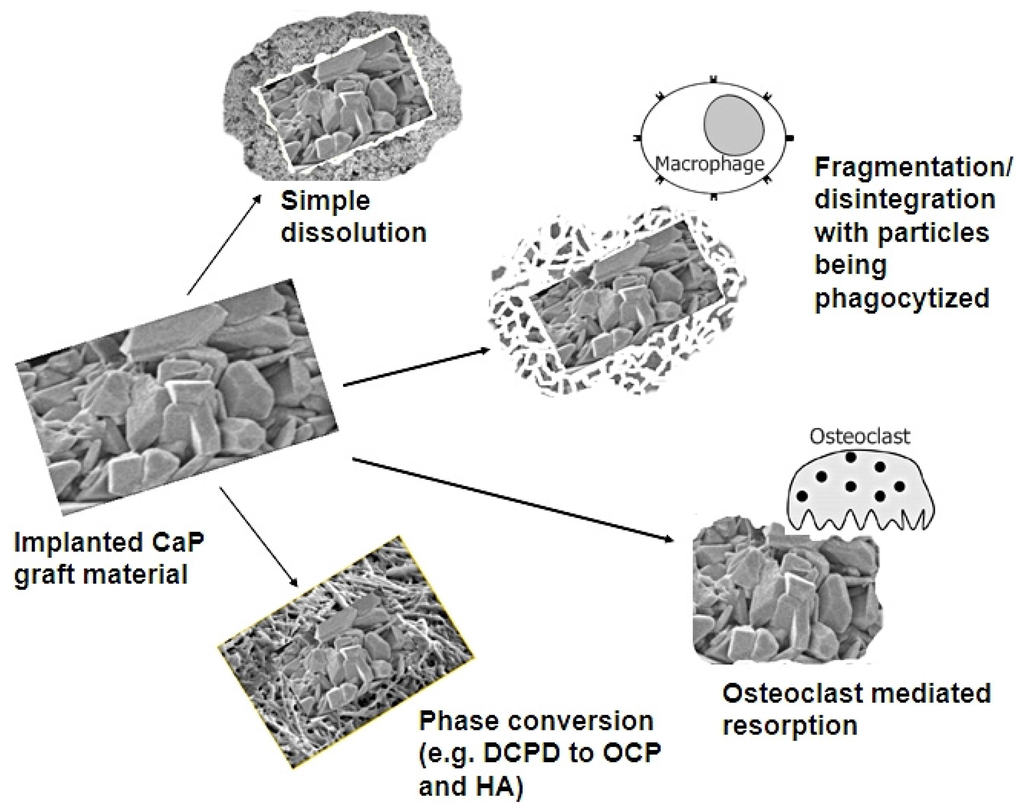
Materials Free FullText Mechanisms of in Vivo Degradation and
What Dissolves Calcium Phosphate As the water is made more. The solubility of calcium salts. In general, ionic substance with anions that are weak bases dissolve better in acidic solution. It is insoluble in ethanol, and acetic. This is the same problem as above. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. Solubility is one of the most important properties of calcium phosphate salts. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? It is the solubility that determines the direction of many reactions that. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. As the water is made more.
From www.alamy.com
3D image of Calcium phosphate skeletal formula molecular chemical What Dissolves Calcium Phosphate This is the same problem as above. It is insoluble in ethanol, and acetic. Solubility is one of the most important properties of calcium phosphate salts. As the water is made more. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. When a slightly soluble ionic compound is added to water, some of it. What Dissolves Calcium Phosphate.
From chemrxiv.org
Pseudoequilibrium equations for calcium phosphate precipitation with What Dissolves Calcium Phosphate What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? This is the same problem as above. It is insoluble in ethanol, and acetic. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. Calcium phosphate appears as a white amorphous or crystalline powder that is. What Dissolves Calcium Phosphate.
From www.youtube.com
How to Dissolve Calcium Deposits in the Body YouTube What Dissolves Calcium Phosphate Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. In general, ionic substance with anions that are weak. What Dissolves Calcium Phosphate.
From formulasense.com
What is Calcium Phosphate? — Formula Sense What Dissolves Calcium Phosphate As the water is made more. This is the same problem as above. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. Solubility is one of the most important properties of calcium phosphate salts. It is insoluble in ethanol,. What Dissolves Calcium Phosphate.
From www.researchgate.net
Agar plate assays show solubilization of calcium phosphate (A) and What Dissolves Calcium Phosphate When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. In general, ionic substance with. What Dissolves Calcium Phosphate.
From courses.lumenlearning.com
Classifying Chemical Reactions CHEM 1305 General Chemistry I—Lecture What Dissolves Calcium Phosphate One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. The solubility of calcium salts. In general, ionic substance with anions that are weak bases dissolve better in acidic solution.. What Dissolves Calcium Phosphate.
From www.mdpi.com
Materials Free FullText Mechanisms of in Vivo Degradation and What Dissolves Calcium Phosphate It is the solubility that determines the direction of many reactions that. It is insoluble in ethanol, and acetic. This is the same problem as above. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. The solubility of calcium salts. Calcium phosphate appears as a white amorphous or. What Dissolves Calcium Phosphate.
From www.researchgate.net
Overview of common calcium phosphate phases Download Scientific Diagram What Dissolves Calcium Phosphate Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. It is insoluble in ethanol, and acetic. It is the solubility that determines the direction of many reactions that. The solubility of calcium salts. In general,. What Dissolves Calcium Phosphate.
From www.semanticscholar.org
Figure 1 from CASEIN PHOSPHOPEPTIDEAMORPHOUS CALCIUM PHOSPHATE What Dissolves Calcium Phosphate One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. Solubility is one of the most important properties of calcium phosphate salts. As the water is made more. It is. What Dissolves Calcium Phosphate.
From www.mdpi.com
IJMS Free FullText Ionic Substitutions in NonApatitic Calcium What Dissolves Calcium Phosphate One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. It is insoluble in ethanol, and acetic. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing. What Dissolves Calcium Phosphate.
From www.researchgate.net
Infrared spectra of precipitated calcium phosphate (a) and What Dissolves Calcium Phosphate As the water is made more. It is the solubility that determines the direction of many reactions that. Solubility is one of the most important properties of calcium phosphate salts. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. In general, ionic substance with anions that are weak bases dissolve. What Dissolves Calcium Phosphate.
From www.researchgate.net
Pathway of calcium phosphate after application as food additive or in What Dissolves Calcium Phosphate It is the solubility that determines the direction of many reactions that. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. Solubility is one of the most important properties of calcium phosphate salts. As the water is made more. The solubility of calcium salts. When a slightly soluble ionic compound. What Dissolves Calcium Phosphate.
From www.mdpi.com
Nanomaterials Free FullText On the Application of Calcium What Dissolves Calcium Phosphate As the water is made more. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. It is insoluble in ethanol, and acetic. In general, ionic substance with anions that are weak bases dissolve better in. What Dissolves Calcium Phosphate.
From www.youtube.com
how to dissolve and prevent calcium phosphate kidney stones with diet What Dissolves Calcium Phosphate One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. The solubility of calcium salts. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. As the water is made more.. What Dissolves Calcium Phosphate.
From healthjade.com
Calcium phosphate uses in food, supplement & calcium phosphate side effects What Dissolves Calcium Phosphate It is the solubility that determines the direction of many reactions that. As the water is made more. What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. Solubility is one of the most important properties of calcium. What Dissolves Calcium Phosphate.
From ar.inspiredpencil.com
Calcium Phosphate Lewis Structure What Dissolves Calcium Phosphate When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. It is the solubility that determines the direction of many reactions that. As the water is made more. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. What is. What Dissolves Calcium Phosphate.
From www.mdpi.com
Nanomaterials Free FullText On the Application of Calcium What Dissolves Calcium Phosphate What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? In general, ionic substance with anions that are weak bases dissolve better in acidic solution. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. One common way to remove phosphates from water is by the. What Dissolves Calcium Phosphate.
From www.researchgate.net
2. SEM images of calcium phosphate coatings formed on various What Dissolves Calcium Phosphate When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. It is insoluble in ethanol, and acetic. It is the solubility that determines the direction of many reactions that. In general, ionic substance with anions that are weak bases dissolve better in acidic solution. Solubility is one of the. What Dissolves Calcium Phosphate.
From www.researchgate.net
Main calcium phosphate (CP) compounds used as bone substitutes and What Dissolves Calcium Phosphate One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. Solubility is one of the most important properties of calcium phosphate salts. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. What is the solubility of calcium phosphate in. What Dissolves Calcium Phosphate.
From www.bartleby.com
Answered Calcium phosphate dissociates in water… bartleby What Dissolves Calcium Phosphate It is the solubility that determines the direction of many reactions that. It is insoluble in ethanol, and acetic. Solubility is one of the most important properties of calcium phosphate salts. The solubility of calcium salts. This is the same problem as above. What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? Calcium phosphates are important. What Dissolves Calcium Phosphate.
From www.slideshare.net
Calcium and phosphate METABOLISM What Dissolves Calcium Phosphate In general, ionic substance with anions that are weak bases dissolve better in acidic solution. This is the same problem as above. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. It is the solubility that determines the direction of many reactions that. It is insoluble in ethanol, and acetic. As the water is. What Dissolves Calcium Phosphate.
From mavink.com
Calcium Phosphate Structure What Dissolves Calcium Phosphate One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. It is insoluble in ethanol, and acetic. This is the same problem as above. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. Calcium phosphates are important materials in. What Dissolves Calcium Phosphate.
From www.numerade.com
SOLVED Calculate the molar solubility of calcium phosphate Ca3(PO4)2 What Dissolves Calcium Phosphate When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. Calcium phosphates are important materials. What Dissolves Calcium Phosphate.
From www.researchgate.net
Solubility isotherms of calcium phosphate phases (A) according to What Dissolves Calcium Phosphate Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. This is the same problem as above. As the water is made more. In general, ionic substance with anions that are weak bases dissolve better in acidic solution. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. Solubility is. What Dissolves Calcium Phosphate.
From fineartamerica.com
Crystal Of Calcium Phosphate In Knee Joint Photograph by Dr Gilbert What Dissolves Calcium Phosphate The solubility of calcium salts. Solubility is one of the most important properties of calcium phosphate salts. What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. It is insoluble in ethanol, and acetic. Calcium phosphate appears as. What Dissolves Calcium Phosphate.
From learnmedicine.org
Calcium and Phosphate Regulation LearnMedicine What Dissolves Calcium Phosphate Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. As the water is made more. This is the same problem as above. Solubility is one of the most important properties of calcium phosphate salts. The solubility of calcium salts. One common way to remove phosphates from water is by the addition of calcium hydroxide,. What Dissolves Calcium Phosphate.
From www.glentham.com
Calcium phosphate tribasic (CAS 12167747) Glentham Life Sciences What Dissolves Calcium Phosphate It is the solubility that determines the direction of many reactions that. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. It is insoluble in ethanol, and acetic. In general, ionic substance with. What Dissolves Calcium Phosphate.
From www.ncbi.nlm.nih.gov
[Figure, Causes of Calcium phosphate deposition flow sheet Prathap What Dissolves Calcium Phosphate One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. It is the solubility that determines the direction of many reactions that. As the water is made more. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. This is. What Dissolves Calcium Phosphate.
From ar.inspiredpencil.com
Calcium Phosphate Lewis Structure What Dissolves Calcium Phosphate Solubility is one of the most important properties of calcium phosphate salts. As the water is made more. It is the solubility that determines the direction of many reactions that. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. It is insoluble in ethanol, and acetic. Calcium phosphate. What Dissolves Calcium Phosphate.
From testbook.com
Calcium Phosphate Definition, Valency, Formula, Properties, Uses What Dissolves Calcium Phosphate Solubility is one of the most important properties of calcium phosphate salts. The solubility of calcium salts. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? Calcium phosphates are important materials in the fields of. What Dissolves Calcium Phosphate.
From www.researchgate.net
Effect of solution pH on the precipitation of calcium phosphate in What Dissolves Calcium Phosphate Solubility is one of the most important properties of calcium phosphate salts. This is the same problem as above. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? It is insoluble in ethanol, and acetic.. What Dissolves Calcium Phosphate.
From solvedlib.com
The molar solubility of calcium phosphate in a water … SolvedLib What Dissolves Calcium Phosphate It is insoluble in ethanol, and acetic. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. It is the solubility that determines the direction of many reactions that. As the water is made more. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. Solubility is one of the. What Dissolves Calcium Phosphate.
From www.researchgate.net
Conversion mechanism of ACP to calcium and phosphate ACP amorphous What Dissolves Calcium Phosphate The solubility of calcium salts. Calcium phosphates are important materials in the fields of biology, geology, industry, medicine, and dentistry. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. One common way to remove phosphates from water is by the addition of calcium hydroxide, or lime, ca(oh) 2. What is the solubility of calcium. What Dissolves Calcium Phosphate.
From www.researchgate.net
Chronological visualization of calcium phosphate solution... Download What Dissolves Calcium Phosphate Solubility is one of the most important properties of calcium phosphate salts. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. It is insoluble in ethanol, and acetic. What is the solubility of calcium phosphate in a 0.100m sodium phosphate solution? In general, ionic substance with anions that. What Dissolves Calcium Phosphate.
From www.numerade.com
SOLVEDSparingly soluble calcium phosphate dissolves in water to yield What Dissolves Calcium Phosphate In general, ionic substance with anions that are weak bases dissolve better in acidic solution. When a slightly soluble ionic compound is added to water, some of it dissolves to form a solution, establishing an equilibrium. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless. Solubility is one of the most important properties of. What Dissolves Calcium Phosphate.