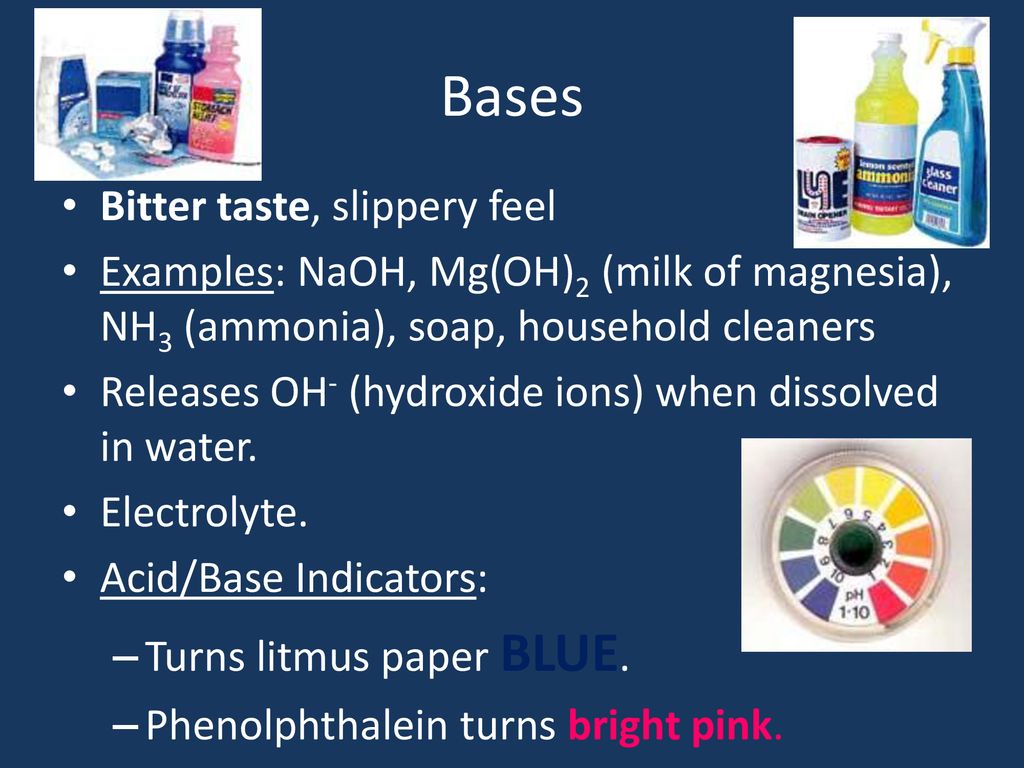
Bitter Bases Examples . Examples of acids and bases; What are acids and bases? Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. Produce a piercing pain in a wound. Base taste bitter has a soapy texture and releases hydroxide ions when dissolved in water. In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. Bases are substances that taste bitter and feel slippery when touched. They are good conductors of electricity and have a ph value of more than seven. Bases, on the other hand, are substances that accept protons or donate electron pairs. There are currently three definitions for acids and bases that entail how they behave when placed in solutions.
from slideplayer.com
If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Bases, on the other hand, are substances that accept protons or donate electron pairs. Bases are substances that taste bitter and feel slippery when touched. What are acids and bases? They are good conductors of electricity and have a ph value of more than seven. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. Examples of acids and bases; Produce a piercing pain in a wound.
Unit 10 Acids & Bases. ppt download
Bitter Bases Examples Bases are substances that taste bitter and feel slippery when touched. Produce a piercing pain in a wound. Bases are substances that taste bitter and feel slippery when touched. Examples of acids and bases; Base taste bitter has a soapy texture and releases hydroxide ions when dissolved in water. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. What are acids and bases? In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. They are good conductors of electricity and have a ph value of more than seven. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. Bases, on the other hand, are substances that accept protons or donate electron pairs.
From slideplayer.com
Unit 10 Acids & Bases. ppt download Bitter Bases Examples There are currently three definitions for acids and bases that entail how they behave when placed in solutions. They are good conductors of electricity and have a ph value of more than seven. Produce a piercing pain in a wound. If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. In. Bitter Bases Examples.
From www.slideserve.com
PPT ACIDS AND BASES PowerPoint Presentation, free download ID2110077 Bitter Bases Examples Produce a piercing pain in a wound. They are good conductors of electricity and have a ph value of more than seven. Base taste bitter has a soapy texture and releases hydroxide ions when dissolved in water. Bases are substances that taste bitter and feel slippery when touched. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and.. Bitter Bases Examples.
From praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Bitter Bases Examples What are acids and bases? Bases, on the other hand, are substances that accept protons or donate electron pairs. If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. They are good conductors of electricity and have a ph value of more than seven. Bases are substances that taste bitter and. Bitter Bases Examples.
From slideplayer.com
Chapter 2 The Chemistry of Life ppt download Bitter Bases Examples Produce a piercing pain in a wound. Bases, on the other hand, are substances that accept protons or donate electron pairs. Bases are substances that taste bitter and feel slippery when touched. In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. Examples of acids and bases; There are currently. Bitter Bases Examples.
From slideplayer.com
Chapters 9 & 19 Chemistry 1K Cypress Creek High School ppt download Bitter Bases Examples Examples of acids and bases; In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. Bases are substances that taste bitter and feel slippery when touched. Bases, on the other hand, are substances that accept protons or donate electron pairs. Produce a piercing pain in a wound. They are good. Bitter Bases Examples.
From slideplayer.com
Biochemistry The Chemistry of Life Text Chapter ppt download Bitter Bases Examples Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. Produce a piercing pain in a wound. Base taste bitter has a soapy texture and releases hydroxide ions when dissolved in water. Examples of acids and bases; There are currently three definitions for acids and bases that entail how they behave when placed in solutions. They are good. Bitter Bases Examples.
From slideplayer.com
Solutions, Solubility and Acids and Bases ppt download Bitter Bases Examples Examples of acids and bases; In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Base taste bitter has a soapy texture and releases hydroxide ions when dissolved in water. Base, in. Bitter Bases Examples.
From slideplayer.com
Unit Twelve Acids and Bases ppt download Bitter Bases Examples What are acids and bases? Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. Examples of acids and bases; In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. There are currently three definitions for acids and bases that entail how they behave when placed in solutions.. Bitter Bases Examples.
From slideplayer.com
Reactions in Aqueous Solution ppt download Bitter Bases Examples There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Examples of acids and bases; Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. Bases, on the other hand, are substances that accept protons or donate electron pairs. They are good conductors of electricity and have a ph value. Bitter Bases Examples.
From slideplayer.com
Think, Pair, Share What can you tell me about acids and bases? ppt download Bitter Bases Examples There are currently three definitions for acids and bases that entail how they behave when placed in solutions. What are acids and bases? Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. Examples of acids. Bitter Bases Examples.
From slideplayer.com
Acids and Bases pH 7 taste sour taste bitter ppt download Bitter Bases Examples There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. Bases, on the other hand, are substances that. Bitter Bases Examples.
From slideplayer.com
Acids and Bases Chapter ppt download Bitter Bases Examples Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. Produce a piercing pain in a wound. Bases are substances that taste bitter and feel slippery when touched. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. Bitter Bases Examples.
From slideplayer.com
Acids and Bases Ch. 8.3 & ppt download Bitter Bases Examples Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. Examples of acids and bases; In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. Bases,. Bitter Bases Examples.
From slideplayer.com
Chapter 16 Acids and Bases ppt download Bitter Bases Examples Produce a piercing pain in a wound. Examples of acids and bases; In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts. Bitter Bases Examples.
From slideplayer.com
Introduction to Acids and Bases ppt download Bitter Bases Examples Bases, on the other hand, are substances that accept protons or donate electron pairs. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. They are good conductors of electricity and have a. Bitter Bases Examples.
From slideplayer.com
Acids and Bases. ppt download Bitter Bases Examples Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. What are acids and bases? Produce a piercing pain in a wound. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. Bases are. Bitter Bases Examples.
From www.slideserve.com
PPT Definitions of Acids and Bases PowerPoint Presentation, free download ID870379 Bitter Bases Examples They are good conductors of electricity and have a ph value of more than seven. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. What are acids and bases? In chemistry, they are known for. Bitter Bases Examples.
From slideplayer.com
7.P.2B.3 Analyze and interpret data to compare the physical properties, chemical properties Bitter Bases Examples Bases are substances that taste bitter and feel slippery when touched. In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. They are good conductors of electricity and have a ph value. Bitter Bases Examples.
From www.reddit.com
The five basic types of tastes r/coolguides Bitter Bases Examples Bases are substances that taste bitter and feel slippery when touched. Bases, on the other hand, are substances that accept protons or donate electron pairs. In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. What are acids and bases? Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide,. Bitter Bases Examples.
From slideplayer.com
Acids and Bases an Introduction ppt download Bitter Bases Examples Examples of acids and bases; Produce a piercing pain in a wound. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids. Bitter Bases Examples.
From slideplayer.com
Solutions, Acids, Bases & pH ppt download Bitter Bases Examples There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. They are good conductors of electricity and have. Bitter Bases Examples.
From www.youtube.com
All bases are bitter in taste. Which of the following substances is a base? CLASS 7 ACIDS, B Bitter Bases Examples In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. They are good conductors of electricity and have a ph value of more than seven. Bases are substances that taste bitter and feel slippery when touched. What are acids and bases? Bases, on the other hand, are substances that accept. Bitter Bases Examples.
From www.slideserve.com
PPT Acid Bases And Salts PowerPoint Presentation, free download ID10217766 Bitter Bases Examples Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. They are good conductors of electricity and have a ph value of more than. Bitter Bases Examples.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID1971171 Bitter Bases Examples If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. Produce a piercing pain in a wound. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and.. Bitter Bases Examples.
From slideplayer.com
Chapter 19 Acids, Bases, and Salts ppt download Bitter Bases Examples If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. Base taste bitter has a soapy texture and releases hydroxide ions when dissolved in water. Bases are substances that taste bitter and feel slippery when touched. Produce a piercing pain in a wound. In chemistry, they are known for turning red. Bitter Bases Examples.
From slideplayer.com
Acids and bases. ppt download Bitter Bases Examples If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. They are good conductors of electricity and have a ph value of more than seven. What are acids and bases? Examples. Bitter Bases Examples.
From slideplayer.com
Physical and Chemical Change ppt download Bitter Bases Examples Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. What are acids and bases? Bases, on. Bitter Bases Examples.
From brainly.in
Some studies have shown that bases are bitter in taste as well as sour. One of the example of Bitter Bases Examples There are currently three definitions for acids and bases that entail how they behave when placed in solutions. What are acids and bases? Produce a piercing pain in a wound. They are good conductors of electricity and have a ph value of more than seven. Examples of acids and bases; Base taste bitter has a soapy texture and releases hydroxide. Bitter Bases Examples.
From slideplayer.com
Unit 12 Acids & Bases ppt download Bitter Bases Examples If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. Examples include baking soda (sodium bicarbonate, nahco₃), soap (sodium hydroxide, naoh), and. Bases are substances that taste bitter and feel slippery when touched. Base taste bitter has a soapy texture and releases hydroxide ions when dissolved in water. Examples of acids. Bitter Bases Examples.
From slideplayer.com
Acids and Bases. ppt download Bitter Bases Examples Examples of acids and bases; In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. Base taste bitter has a soapy texture and releases hydroxide ions when dissolved in water. Bases,. Bitter Bases Examples.
From slidetodoc.com
CHAPTER 5 ACIDS BASES AND SALTS 1 Tastes Bitter Bases Examples Examples of acids and bases; They are good conductors of electricity and have a ph value of more than seven. Bases, on the other hand, are substances that accept protons or donate electron pairs. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper. Bitter Bases Examples.
From slideplayer.com
3.2 Acid and Bases. ppt download Bitter Bases Examples Bases are substances that taste bitter and feel slippery when touched. Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. Bases, on the other hand, are substances that accept protons or donate electron pairs. There. Bitter Bases Examples.
From www.slideserve.com
PPT Chapter 4 Types of Chemical Reactions and Solution Stoichiometry PowerPoint Presentation Bitter Bases Examples In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. Base taste bitter has a soapy texture and releases hydroxide ions when dissolved in water. There are currently three definitions for acids and bases that entail how they behave when placed in solutions. Examples include baking soda (sodium bicarbonate, nahco₃),. Bitter Bases Examples.
From slideplayer.com
Atoms, Elements and How They Behave ppt download Bitter Bases Examples Examples of acids and bases; If you've tasted stuff with too much baking soda in them, well, they taste bitter because bases taste bitter. They are good conductors of electricity and have a ph value of more than seven. In chemistry, they are known for turning red litmus paper blue and are commonly found in household items like baking. Base. Bitter Bases Examples.
From slideplayer.com
Acids and Bases Chapters 14 and ppt download Bitter Bases Examples Base, in chemistry, any substance that in water solution is slippery to the touch, tastes bitter, changes the color of indicators (e.g., turns red litmus paper blue), reacts with acids to form salts, and. Examples of acids and bases; They are good conductors of electricity and have a ph value of more than seven. Bases are substances that taste bitter. Bitter Bases Examples.