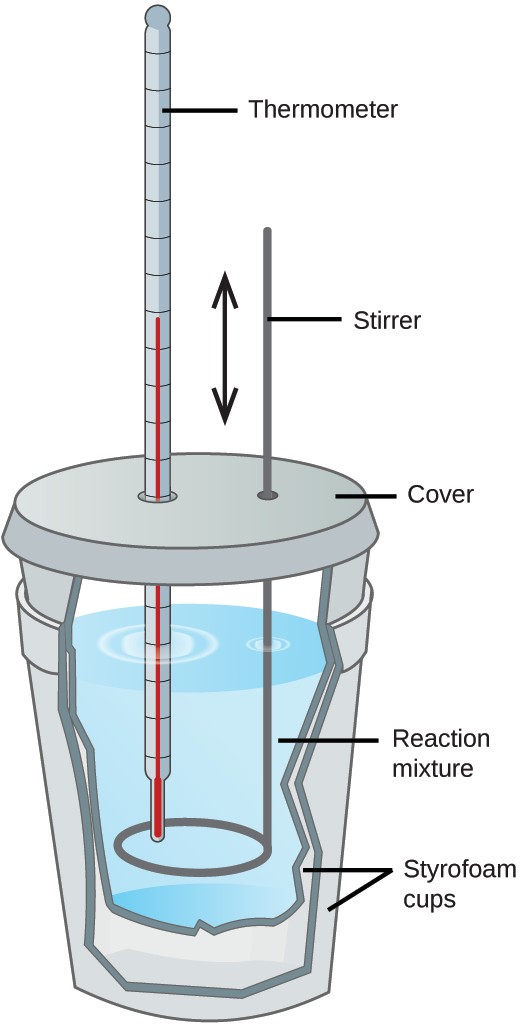
Calorimetry In Chemistry Pdf . the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. lab session 9, experiment 8: The heat evolved by a chemical reaction can be determined using a calorimeter. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. first law, calorimetry, enthalpy monday, january 23 chem 102h t. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a.
from louis.pressbooks.pub
the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. lab session 9, experiment 8: Hughbanks calorimetry reactions are usually done at either constant v (in a closed. The heat evolved by a chemical reaction can be determined using a calorimeter. first law, calorimetry, enthalpy monday, january 23 chem 102h t. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a.
Calorimetry (9.2) General Chemistry
Calorimetry In Chemistry Pdf first law, calorimetry, enthalpy monday, january 23 chem 102h t. the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. first law, calorimetry, enthalpy monday, january 23 chem 102h t. The heat evolved by a chemical reaction can be determined using a calorimeter. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. lab session 9, experiment 8: calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a.
From fyokoklvl.blob.core.windows.net
Calorimetry Definition In Chemistry at Patrick McBean blog Calorimetry In Chemistry Pdf calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. lab session 9, experiment 8:. Calorimetry In Chemistry Pdf.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry In Chemistry Pdf first law, calorimetry, enthalpy monday, january 23 chem 102h t. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. lab session 9, experiment 8: Hughbanks calorimetry reactions are usually done at either constant v (in a closed. Specific heat is an intensive property of a single phase (solid, liquid. Calorimetry In Chemistry Pdf.
From br.pinterest.com
Calorimetry Notes Chemistry notes, Chemistry lessons, Study chemistry Calorimetry In Chemistry Pdf Hughbanks calorimetry reactions are usually done at either constant v (in a closed. first law, calorimetry, enthalpy monday, january 23 chem 102h t. lab session 9, experiment 8: calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. calorimetry is the process of measuring the amount of heat released. Calorimetry In Chemistry Pdf.
From www.animalia-life.club
Calorimeter Diagram Calorimetry In Chemistry Pdf lab session 9, experiment 8: The heat evolved by a chemical reaction can be determined using a calorimeter. first law, calorimetry, enthalpy monday, january 23 chem 102h t. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. the ease of measurement of energy changes in calories has meant. Calorimetry In Chemistry Pdf.
From www.wizeprep.com
Calorimetry Wize University Chemistry Textbook Wizeprep Calorimetry In Chemistry Pdf The heat evolved by a chemical reaction can be determined using a calorimeter. lab session 9, experiment 8: calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. first law, calorimetry, enthalpy monday, january 23 chem 102h t. the ease of measurement of energy changes in calories has meant. Calorimetry In Chemistry Pdf.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry In Chemistry Pdf calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. lab session 9, experiment 8: the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. first law, calorimetry, enthalpy monday, january 23 chem 102h t. The heat evolved by a chemical. Calorimetry In Chemistry Pdf.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry In Chemistry Pdf Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. first law, calorimetry, enthalpy monday, january 23 chem 102h t. The heat evolved by a chemical reaction can be determined using a calorimeter. the ease of measurement of. Calorimetry In Chemistry Pdf.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu Calorimetry In Chemistry Pdf the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. lab session 9, experiment 8: Hughbanks calorimetry reactions are usually done at either constant v (in a closed. calculate heat, temperature change,. Calorimetry In Chemistry Pdf.
From www.studocu.com
Calorimetry Intro Calorimetry In every chemical reaction that occurs Calorimetry In Chemistry Pdf the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. The heat evolved by a chemical reaction can be determined using a calorimeter. lab session 9, experiment 8: first law, calorimetry, enthalpy monday, january 23 chem 102h t. Hughbanks calorimetry reactions are usually done at either constant v (in. Calorimetry In Chemistry Pdf.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry In Chemistry Pdf first law, calorimetry, enthalpy monday, january 23 chem 102h t. lab session 9, experiment 8: the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Specific heat is an intensive property. Calorimetry In Chemistry Pdf.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry In Chemistry Pdf calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. calculate heat, temperature change, and specific heat after thermal equilibrium is. Calorimetry In Chemistry Pdf.
From www.academia.edu
(PDF) Bomb Calorimetry Experiment Bryle Kristiann Camarote Academia.edu Calorimetry In Chemistry Pdf first law, calorimetry, enthalpy monday, january 23 chem 102h t. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a.. Calorimetry In Chemistry Pdf.
From hxeenedvw.blob.core.windows.net
Calorimeter Chemistry Ia at Beverly McPhee blog Calorimetry In Chemistry Pdf Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. lab session 9, experiment 8: first law, calorimetry, enthalpy monday, january 23 chem 102h t. Hughbanks calorimetry reactions are usually done at either. Calorimetry In Chemistry Pdf.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimetry In Chemistry Pdf first law, calorimetry, enthalpy monday, january 23 chem 102h t. The heat evolved by a chemical reaction can be determined using a calorimeter. the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical. Calorimetry In Chemistry Pdf.
From www.thinkswap.com
Chemistry Notes Calorimetry and Writing a Report Chemistry Year 11 Calorimetry In Chemistry Pdf the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes.. Calorimetry In Chemistry Pdf.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry In Chemistry Pdf first law, calorimetry, enthalpy monday, january 23 chem 102h t. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. The heat evolved by a chemical reaction can be determined using a calorimeter. calculate heat, temperature change, and. Calorimetry In Chemistry Pdf.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry In Chemistry Pdf lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. The heat evolved by a chemical reaction can be determined using a calorimeter. calorimetry is the process of measuring the amount of heat. Calorimetry In Chemistry Pdf.
From www.studocu.com
Calorimetry Lab Experiment 25 Calorimetry Data Unknown Calcium Calorimetry In Chemistry Pdf The heat evolved by a chemical reaction can be determined using a calorimeter. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. first law, calorimetry, enthalpy monday, january 23 chem 102h t. lab session 9, experiment 8: calorimetry is the process of measuring the amount of heat released or absorbed during a chemical. Calorimetry In Chemistry Pdf.
From fyocdslfs.blob.core.windows.net
Calorimetry Explained at Edith Wallis blog Calorimetry In Chemistry Pdf calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. The heat evolved by a. Calorimetry In Chemistry Pdf.
From www.studocu.com
Chem 112 Calorimetry Pdf CALORIMETRY INTRODUCTION The heat evolved Calorimetry In Chemistry Pdf calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. The heat evolved by a chemical reaction can be determined using a calorimeter. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Hughbanks calorimetry reactions are usually done at either constant v (in a. Calorimetry In Chemistry Pdf.
From exyqlzddm.blob.core.windows.net
Calorimeter Examples Chemistry at Sima Montgomery blog Calorimetry In Chemistry Pdf calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. first law, calorimetry, enthalpy monday, january 23 chem 102h t. the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. Specific heat is an intensive property of a single phase (solid, liquid. Calorimetry In Chemistry Pdf.
From www.w3schools.blog
Cp, Cv calorimetry W3schools Calorimetry In Chemistry Pdf lab session 9, experiment 8: The heat evolved by a chemical reaction can be determined using a calorimeter. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. the ease of measurement of energy changes in calories. Calorimetry In Chemistry Pdf.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry In Chemistry Pdf Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. lab session 9, experiment 8: calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. the ease of measurement of energy changes in calories has meant that the calorie is still frequently used.. Calorimetry In Chemistry Pdf.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry In Chemistry Pdf calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. calculate heat, temperature change, and. Calorimetry In Chemistry Pdf.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry In Chemistry Pdf the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. The heat evolved by a chemical reaction can be determined using a calorimeter. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. first law, calorimetry, enthalpy monday, january 23 chem 102h t. lab session 9,. Calorimetry In Chemistry Pdf.
From chem.libretexts.org
6.3 Calorimetry Chemistry LibreTexts Calorimetry In Chemistry Pdf Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. lab session 9, experiment 8: The heat evolved by a chemical reaction can be determined using a calorimeter. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. the ease of measurement of. Calorimetry In Chemistry Pdf.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry In Chemistry Pdf Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. Hughbanks calorimetry reactions are usually done at. Calorimetry In Chemistry Pdf.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry Calorimetry In Chemistry Pdf The heat evolved by a chemical reaction can be determined using a calorimeter. first law, calorimetry, enthalpy monday, january 23 chem 102h t. the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical. Calorimetry In Chemistry Pdf.
From dokumen.tips
(PDF) Thermochemistry and calorimetry · PDF fileheat and enthalpy Calorimetry In Chemistry Pdf calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. The heat evolved by a chemical reaction can be determined using a calorimeter. first law, calorimetry, enthalpy monday, january 23 chem 102h t. the ease of measurement of energy changes in calories has meant that the calorie is still frequently. Calorimetry In Chemistry Pdf.
From vdocuments.mx
Chemistry 11 Notes on Heat and Calorimetry D · PDF fileChemistry 11 Calorimetry In Chemistry Pdf calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. first law, calorimetry, enthalpy monday, january 23 chem 102h t. lab session 9, experiment 8: Hughbanks calorimetry reactions are usually done at. Calorimetry In Chemistry Pdf.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Calorimetry In Chemistry Pdf first law, calorimetry, enthalpy monday, january 23 chem 102h t. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Hughbanks calorimetry reactions are usually done at either constant v (in a closed. lab session 9, experiment 8: the ease of measurement of energy changes in calories has meant. Calorimetry In Chemistry Pdf.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry In Chemistry Pdf Hughbanks calorimetry reactions are usually done at either constant v (in a closed. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. lab session 9, experiment 8: calculate heat, temperature change,. Calorimetry In Chemistry Pdf.
From studylib.net
Calorimetry Chemistry 301 Calorimetry In Chemistry Pdf Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. The heat evolved by a chemical reaction can be determined using a calorimeter. the ease of measurement of energy changes in calories has meant that the calorie is still frequently used. lab session 9, experiment 8: calculate heat, temperature change,. Calorimetry In Chemistry Pdf.
From chem.libretexts.org
6.3 Calorimetry Chemistry LibreTexts Calorimetry In Chemistry Pdf The heat evolved by a chemical reaction can be determined using a calorimeter. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. the ease of measurement of energy changes in calories has meant. Calorimetry In Chemistry Pdf.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Calorimetry In Chemistry Pdf The heat evolved by a chemical reaction can be determined using a calorimeter. lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. the ease of measurement of. Calorimetry In Chemistry Pdf.