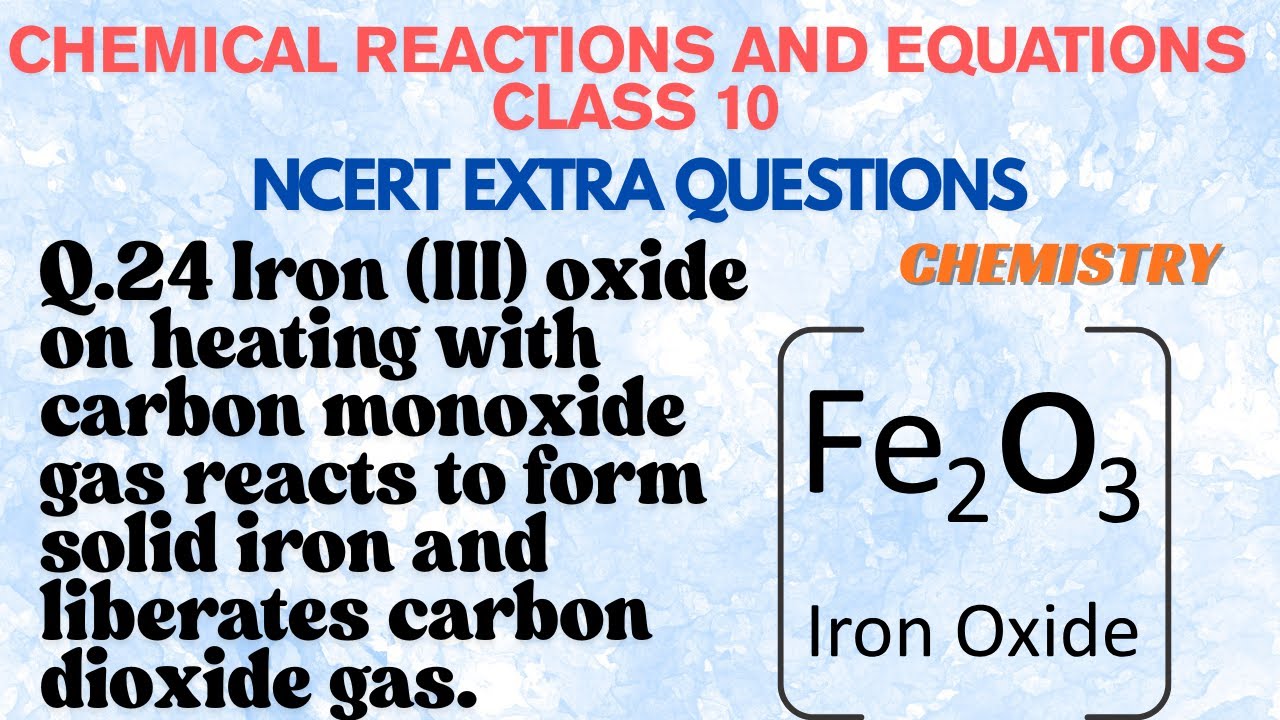Iron Iii Oxide + Carbon . iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. iron (iii) oxide reacts with carbon monoxide according to the equation: The experiment provides a quick example of metal. iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. to balance fe2o3 + co = fe + co2 you'll need to watch out for two.
from www.youtube.com
iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. The experiment provides a quick example of metal. iron (iii) oxide reacts with carbon monoxide according to the equation: Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture.
Iron (III) oxide on heating with carbon monoxide gas reacts to form
Iron Iii Oxide + Carbon Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. The experiment provides a quick example of metal. iron (iii) oxide reacts with carbon monoxide according to the equation:
From www.slideserve.com
PPT Stoichiometry with a Twist PowerPoint Presentation ID1836706 Iron Iii Oxide + Carbon Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. iron(iii) oxide react with carbon monoxide to. Iron Iii Oxide + Carbon.
From slideplayer.com
Calculations Based on Chemical Equations ppt download Iron Iii Oxide + Carbon Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron (iii) oxide reacts with carbon monoxide according to the equation: iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. The. Iron Iii Oxide + Carbon.
From slideplayer.com
(D) OXIDISING AND REDUCING AGENTS ppt download Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. The experiment provides a quick example of metal.. Iron Iii Oxide + Carbon.
From stock.adobe.com
iron III Oxide Properties and Chemical Compound Structure Stock Vector Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide according to the equation: Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide. Iron Iii Oxide + Carbon.
From www.coursehero.com
[Solved] Iron(III) oxide reacts with carbon monoxide to produce Iron Iii Oxide + Carbon Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. The experiment provides a quick example of metal. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. to. Iron Iii Oxide + Carbon.
From www.chegg.com
Solved ? iron (III) oxide + ? carbon monoxide iron (II) Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide according to the equation: The experiment provides a quick example of metal. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron(iii) oxide react with carbon monoxide. Iron Iii Oxide + Carbon.
From giorvjwuc.blob.core.windows.net
Iron Iii Oxide Oxidation Number at Agnes McKim blog Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide according to the equation: iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. . Iron Iii Oxide + Carbon.
From www.toppr.com
Iron III Oxide Formula Definition, Concepts and Examples Iron Iii Oxide + Carbon to balance fe2o3 + co = fe + co2 you'll need to watch out for two. iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. iron (iii) oxide reacts with carbon monoxide. Iron Iii Oxide + Carbon.
From www.numerade.com
SOLVEDIron(III) oxide reacts with carbon monoxide according to the Iron Iii Oxide + Carbon iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. to balance fe2o3 + c = fe + co2 you'll. Iron Iii Oxide + Carbon.
From www.toppr.com
The balanced chemical equation for the reaction of iron (III) oxide and Iron Iii Oxide + Carbon iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. iron (iii) oxide reacts with carbon monoxide to form solid. Iron Iii Oxide + Carbon.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron Iii Oxide + Carbon in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. The experiment provides a quick example of metal. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. . Iron Iii Oxide + Carbon.
From www.numerade.com
SOLVED47. Iron(III) oxide reacts with carbon monoxide equation Iron Iii Oxide + Carbon in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. iron(iii) oxide react with carbon. Iron Iii Oxide + Carbon.
From kunduz.com
[ANSWERED] Iron (III) oxide reacts with carbon monoxide according to Iron Iii Oxide + Carbon Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. to balance fe2o3 + c = fe + co2 you'll. Iron Iii Oxide + Carbon.
From www.studypool.com
SOLUTION Iron(III) oxide reacts with carbon monoxide according to the Iron Iii Oxide + Carbon iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. The experiment provides a quick example of metal. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. to balance fe2o3. Iron Iii Oxide + Carbon.
From www.numerade.com
SOLVED Iron (III) oxide reacts with carbon to give iron and carbon Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: The experiment provides a quick example of metal. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. Fe 2. Iron Iii Oxide + Carbon.
From www.slideshare.net
Chemical Reactions Iron Iii Oxide + Carbon iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: to balance fe2o3 + co = fe + co2 you'll need to watch out for two. to balance fe2o3 + c = fe + co2 you'll. Iron Iii Oxide + Carbon.
From solvedlib.com
Iron(III) oxide reacts with carbon to give iron and c… SolvedLib Iron Iii Oxide + Carbon iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. The experiment provides a quick example of metal. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron (iii) oxide reacts with. Iron Iii Oxide + Carbon.
From www.chegg.com
Solved 3. Iron(III) oxide reacts with carbon monoxide to Iron Iii Oxide + Carbon iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. iron(iii) oxide react with carbon monoxide. Iron Iii Oxide + Carbon.
From www.numerade.com
Iron(III) oxide reacts with carbon monoxide according to the equation Iron Iii Oxide + Carbon Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. Fe 2 o 3 ( s ) +. Iron Iii Oxide + Carbon.
From www.slideserve.com
PPT 29 When 84.8 g of iron (III) oxide reacts with excess of carbon Iron Iii Oxide + Carbon Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. iron (iii) oxide reacts with carbon monoxide according to the equation: Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g). Iron Iii Oxide + Carbon.
From www.numerade.com
SOLVED Iron (III) oxide reacts with carbon monoxide to produce iron Iron Iii Oxide + Carbon Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. iron (iii) oxide reacts with carbon monoxide according to the equation: iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. in this experiment, students reduce iron (iii) oxide with carbon on a match head to. Iron Iii Oxide + Carbon.
From www.coursehero.com
[Solved] Iron (III) oxide reacts with carbon monoxide to produce Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: The experiment provides a quick example of metal. Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. iron (iii) oxide reacts with carbon monoxide according to the equation: in this experiment, students. Iron Iii Oxide + Carbon.
From www.chegg.com
Solved ? iron (III) oxide + ? carbon monoxide iron (II) Iron Iii Oxide + Carbon The experiment provides a quick example of metal. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. Fe. Iron Iii Oxide + Carbon.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. iron(iii) oxide can be. Iron Iii Oxide + Carbon.
From www.slideserve.com
PPT 29 When 84.8 g of iron (III) oxide reacts with excess of carbon Iron Iii Oxide + Carbon Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. iron (iii) oxide reacts with carbon monoxide to form. Iron Iii Oxide + Carbon.
From www.youtube.com
Iron (III) oxide on heating with carbon monoxide gas reacts to form Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide according to the equation: iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate.. Iron Iii Oxide + Carbon.
From www.chegg.com
Solved When iron(III) oxide reacts with carbon monoxide it Iron Iii Oxide + Carbon iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. iron (iii) oxide reacts with carbon monoxide according to the equation: iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide. Iron Iii Oxide + Carbon.
From www.chegg.com
Solved ? iron (III) oxide + ? carbon monox Iron(III) oxide Iron Iii Oxide + Carbon The experiment provides a quick example of metal. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. in this experiment, students reduce iron (iii) oxide with carbon on a match head to. Iron Iii Oxide + Carbon.
From www.youtube.com
Iron (III) oxide on heating with carbon monoxide gas reacts to form Iron Iii Oxide + Carbon iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: The experiment provides a quick example of metal.. Iron Iii Oxide + Carbon.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron Iii Oxide + Carbon in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. Fe 2 o 3 ( s ) + 3 co ( g ) → 2 fe (. iron (iii) oxide reacts with carbon monoxide. Iron Iii Oxide + Carbon.
From www.showme.com
ShowMe iron Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide according to the equation: iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. The experiment provides a quick example of metal. in this experiment, students reduce iron (iii) oxide with. Iron Iii Oxide + Carbon.
From slideplayer.com
Calculations Based on Chemical Equations ppt download Iron Iii Oxide + Carbon Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. The experiment provides a quick example of metal. to balance fe2o3 + co = fe + co2 you'll need to watch out for two. in this experiment, students reduce iron (iii) oxide with carbon on a match head to produce iron. . Iron Iii Oxide + Carbon.
From www.studypool.com
SOLUTION Iron(III) oxide reacts with carbon monoxide according to the Iron Iii Oxide + Carbon The experiment provides a quick example of metal. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of. iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. Fe2o3 (s) + 3 co (g)¡2 fe (s) + 3 co2 ( g) a reaction mixture. . Iron Iii Oxide + Carbon.
From www.numerade.com
SOLVED Iron metal is produced commercially by reducing iron (III Iron Iii Oxide + Carbon iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: iron (iii) oxide reacts with carbon monoxide according to the equation: to balance fe2o3 + co = fe + co2 you'll need to watch out for two. iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide. Iron Iii Oxide + Carbon.
From www.slideserve.com
PPT What is the difference between a chemical reaction and physical Iron Iii Oxide + Carbon iron(iii) oxide can be formed from the thermal decomposition of iron(iii) hydroxide and iron(ii) sulfate. iron (iii) oxide reacts with carbon monoxide to form solid iron and carbon dioxide gas as follows: to balance fe2o3 + co = fe + co2 you'll need to watch out for two. Fe 2 o 3 ( s ) + 3. Iron Iii Oxide + Carbon.