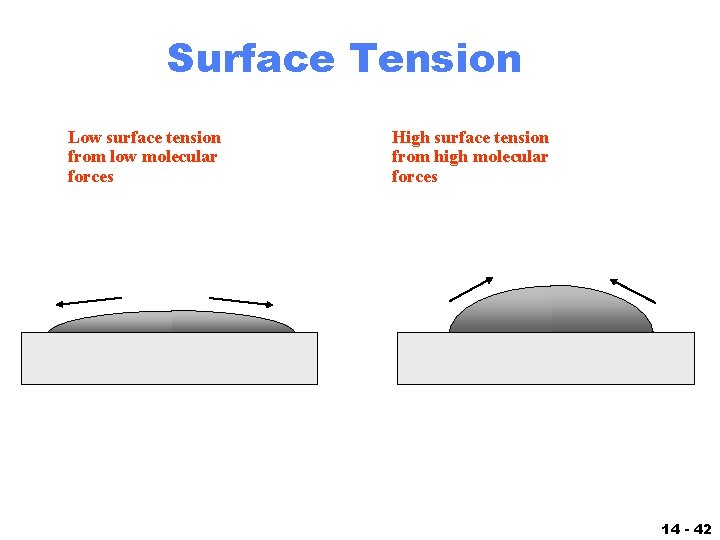
Lowest Surface Tension Molecule . How does polarity affect it. what is surface tension. Equivalently, it can be stated as surface energy in ergs. Learn the surface tension of water. Check out a few diagrams. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values.
from slidetodoc.com
How does polarity affect it. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. Check out a few diagrams. what is surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Learn the surface tension of water. Equivalently, it can be stated as surface energy in ergs.
Lecture 14 Taken in part from Chapters 13
Lowest Surface Tension Molecule How does polarity affect it. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. what is surface tension. How does polarity affect it. Check out a few diagrams. Learn the surface tension of water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. Equivalently, it can be stated as surface energy in ergs.
From www.researchgate.net
The role of a low surface tension liquid on the droplet wetting state Lowest Surface Tension Molecule Equivalently, it can be stated as surface energy in ergs. Learn the surface tension of water. How does polarity affect it. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Check out a few diagrams. what is surface tension. for example, water, with its strong intermolecular. Lowest Surface Tension Molecule.
From byjus.com
Explain the surface tension phenomenon with examples. Lowest Surface Tension Molecule for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. surface tension. Lowest Surface Tension Molecule.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Lowest Surface Tension Molecule for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. How does polarity affect it. Learn the surface tension of water. what is surface tension. surface tension is typically measured in dynes/cm, the force in. Lowest Surface Tension Molecule.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Lowest Surface Tension Molecule surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. what is surface tension. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. Learn the surface tension of water. Equivalently, it can be stated as surface energy in ergs.. Lowest Surface Tension Molecule.
From fyoppgora.blob.core.windows.net
Less Surface Tension Means at Stephanie Robertson blog Lowest Surface Tension Molecule liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. what is surface tension. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. Check out a few diagrams. How does polarity affect it. surface tension is the energy, or work, required to increase the. Lowest Surface Tension Molecule.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free Lowest Surface Tension Molecule in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. Learn the surface tension of water. what is surface tension. Check out a few diagrams. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Equivalently, it can be stated. Lowest Surface Tension Molecule.
From www.youtube.com
Surface Tension of Water Explained YouTube Lowest Surface Tension Molecule in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. what is. Lowest Surface Tension Molecule.
From readchemistry.com
Determination of Surface Tension Read Chemistry Lowest Surface Tension Molecule liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Check out a few diagrams. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. How. Lowest Surface Tension Molecule.
From www.pinterest.com
Surface tension, Surface, Tension Lowest Surface Tension Molecule in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. what is surface tension. Learn the surface tension of water. How does polarity affect it. surface tension is the energy, or work, required to increase. Lowest Surface Tension Molecule.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Lowest Surface Tension Molecule How does polarity affect it. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Equivalently, it can be stated as surface energy in ergs. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. for example, water, with its. Lowest Surface Tension Molecule.
From byjus.com
Explain surface tension on the basis of molecular theory. Lowest Surface Tension Molecule Learn the surface tension of water. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. How does polarity affect it. what is surface tension. Check out a few diagrams.. Lowest Surface Tension Molecule.
From slidetodoc.com
Lecture 14 Taken in part from Chapters 13 Lowest Surface Tension Molecule Equivalently, it can be stated as surface energy in ergs. Check out a few diagrams. what is surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length. Lowest Surface Tension Molecule.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Lowest Surface Tension Molecule for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. what is surface tension. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. Learn. Lowest Surface Tension Molecule.
From fyojeklzt.blob.core.windows.net
How Does The Surface Tension Of Water Compare To Other Liquids at Lowest Surface Tension Molecule Learn the surface tension of water. what is surface tension. Check out a few diagrams. Equivalently, it can be stated as surface energy in ergs. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. surface tension is the energy, or work, required to increase the surface area of a. Lowest Surface Tension Molecule.
From www.youtube.com
Surface Tension on the basis of Molecular theory YouTube Lowest Surface Tension Molecule what is surface tension. Learn the surface tension of water. Check out a few diagrams. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. liquids with high surface. Lowest Surface Tension Molecule.
From giobkbnor.blob.core.windows.net
Water Molecule Surface Tension Property at Virginia Sherman blog Lowest Surface Tension Molecule surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. what is surface tension. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. Equivalently, it can be stated as surface energy in ergs. Learn the surface tension of water.. Lowest Surface Tension Molecule.
From www.slideserve.com
PPT Chapter 5 Liquids and Solids PowerPoint Presentation, free Lowest Surface Tension Molecule Check out a few diagrams. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. what is surface tension. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Equivalently, it can be stated as surface energy in ergs. How does polarity affect it. surface. Lowest Surface Tension Molecule.
From www.vrogue.co
What Is Surface Tension Molecular Explanation vrogue.co Lowest Surface Tension Molecule in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Equivalently, it can be stated as surface energy in ergs. How does polarity affect it. Check out a few diagrams. Learn the surface tension of water. . Lowest Surface Tension Molecule.
From www.researchgate.net
ntro19 A molecule of the liquid having the lowest surface tension Lowest Surface Tension Molecule in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Check out a few diagrams. Learn. Lowest Surface Tension Molecule.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Lowest Surface Tension Molecule liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Equivalently, it can be stated as surface energy in ergs. Check out a few diagrams. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. what is surface tension. surface tension is typically measured in. Lowest Surface Tension Molecule.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download Lowest Surface Tension Molecule surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Check out a few diagrams. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. . Lowest Surface Tension Molecule.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and Lowest Surface Tension Molecule Check out a few diagrams. Equivalently, it can be stated as surface energy in ergs. Learn the surface tension of water. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension is the energy, or work, required to increase the surface area of a liquid due. Lowest Surface Tension Molecule.
From chem.libretexts.org
7.1 Surface Tension, Viscosity, and Capillary Action (Problems Lowest Surface Tension Molecule Check out a few diagrams. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. liquids with high surface tension exhibit significant resistance to penetration compared to. Lowest Surface Tension Molecule.
From www.chegg.com
Solved Which molecule has the lowest surface tension? Lowest Surface Tension Molecule Equivalently, it can be stated as surface energy in ergs. How does polarity affect it. Check out a few diagrams. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. what is surface tension. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. in. Lowest Surface Tension Molecule.
From www.chegg.com
Solved Arrange the following compounds from lowest surface Lowest Surface Tension Molecule How does polarity affect it. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. Learn the surface tension of water. what is surface tension. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. liquids with high surface. Lowest Surface Tension Molecule.
From www.researchgate.net
Surfactant provides low surface tension and anti invader cover to Lowest Surface Tension Molecule How does polarity affect it. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. what is surface tension. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. liquids with high surface tension exhibit significant resistance to penetration. Lowest Surface Tension Molecule.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Lowest Surface Tension Molecule surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. what is surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Learn the surface tension of water. in comparison, organic liquids, such as. Lowest Surface Tension Molecule.
From universe-review.ca
Molecules Lowest Surface Tension Molecule How does polarity affect it. Check out a few diagrams. Equivalently, it can be stated as surface energy in ergs. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. what is surface tension. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1. Lowest Surface Tension Molecule.
From www.acs.org
Simulations & Videos for Lesson 5.2 Surface Tension American Lowest Surface Tension Molecule Learn the surface tension of water. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. what is surface tension. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. liquids with high surface tension exhibit significant resistance to. Lowest Surface Tension Molecule.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Lowest Surface Tension Molecule in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. Check out a few diagrams. surface tension is typically measured in dynes/cm, the force in dynes required to break a film of length 1 cm. How does polarity affect it. liquids with high surface tension exhibit significant resistance to penetration. Lowest Surface Tension Molecule.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter Lowest Surface Tension Molecule surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Check out a few diagrams. Equivalently, it can be stated as surface energy in ergs. How does polarity affect it. what is surface tension. surface tension is typically measured in dynes/cm, the force in dynes required to. Lowest Surface Tension Molecule.
From www.chegg.com
Solved Which molecule has the lowest surface tension? Lowest Surface Tension Molecule Check out a few diagrams. what is surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions,. Lowest Surface Tension Molecule.
From giofsecel.blob.core.windows.net
What Properties Does Surface Tension Itself Affect at Jon blog Lowest Surface Tension Molecule for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. what is surface tension. liquids with high surface tension exhibit significant resistance to penetration compared to the resistance. Check out a few diagrams. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has. Lowest Surface Tension Molecule.
From mavink.com
Water Molecules Surface Tension Lowest Surface Tension Molecule How does polarity affect it. Check out a few diagrams. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Learn the surface tension of water. liquids with high surface. Lowest Surface Tension Molecule.
From www.chegg.com
Solved Which molecule has the lowest surface tension? Lowest Surface Tension Molecule what is surface tension. in comparison, organic liquids, such as benzene and alcohols, have lower surface tensions, whereas mercury has a. for example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values. Equivalently, it can be stated as surface energy in ergs. surface tension is the energy, or work, required. Lowest Surface Tension Molecule.