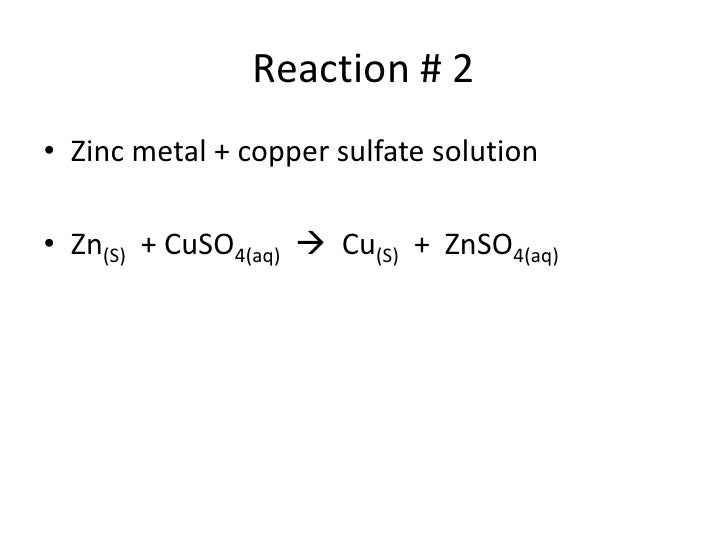
Magnesium And Zinc Sulfate Balanced Equation . Zn + mgso_4 mg + znso_4 plan: Explain the roles of subscripts and coefficients in chemical equations. Derive chemical equations from narrative descriptions of chemical reactions. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Zinc + magnesium sulfate = magnesium + zinc sulfate. The balanced chemical equation for this reaction is: • determine the stoichiometric numbers. Balance a chemical equation when given the unbalanced equation. • assemble the rate term for. Construct the rate of reaction expression for: Zn + mgso4 = mg + zn(so4) is a single displacement (substitution). Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: • balance the chemical equation. There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are.
from www.slideshare.net
There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. • balance the chemical equation. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: Balance a chemical equation when given the unbalanced equation. Derive chemical equations from narrative descriptions of chemical reactions. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Construct the rate of reaction expression for: Zn + mgso_4 mg + znso_4 plan: The balanced chemical equation for this reaction is: • assemble the rate term for.
Replacement reactions
Magnesium And Zinc Sulfate Balanced Equation Zn + mgso4 = mg + zn(so4) is a single displacement (substitution). Zn + mgso4 = mg + zn(so4) is a single displacement (substitution). • determine the stoichiometric numbers. Write and balance chemical equations in molecular,. The balanced chemical equation for this reaction is: Balance a chemical equation when given the unbalanced equation. Derive chemical equations from narrative descriptions of chemical reactions. Zinc + magnesium sulfate = magnesium + zinc sulfate. Explain the roles of subscripts and coefficients in chemical equations. Zn + mgso_4 mg + znso_4 plan: There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: Construct the rate of reaction expression for: Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. • balance the chemical equation. • assemble the rate term for.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Chemical For Zinc Sulfuric Magnesium And Zinc Sulfate Balanced Equation Construct the rate of reaction expression for: Write and balance chemical equations in molecular,. Derive chemical equations from narrative descriptions of chemical reactions. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: • assemble the rate term for. Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given. Magnesium And Zinc Sulfate Balanced Equation.
From www.chegg.com
Solved Write A Balanced Chemical Equation For Each Of The... Magnesium And Zinc Sulfate Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Construct the rate of reaction expression for: • determine the stoichiometric numbers. • assemble the rate term for. Zinc + magnesium sulfate = magnesium + zinc sulfate. • balance the chemical equation. Explain the roles of subscripts. Magnesium And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED Similar to magnesium, zinc also reacts with hydrochloric acid to form hydrogen gas Magnesium And Zinc Sulfate Balanced Equation Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The balanced chemical equation for this reaction is: • assemble the rate term for. • determine the stoichiometric numbers. Balance a chemical equation when given the unbalanced equation. Zn + mgso₄ → znso₄ + mg let's break down this equation. Magnesium And Zinc Sulfate Balanced Equation.
From brainly.in
write the balanced chemical equation for the reaction that takes place when zinc magnesium react Magnesium And Zinc Sulfate Balanced Equation Zn + mgso4 = mg + zn(so4) is a single displacement (substitution). Explain the roles of subscripts and coefficients in chemical equations. Balance a chemical equation when given the unbalanced equation. Zn + mgso_4 mg + znso_4 plan: • balance the chemical equation. Zinc + magnesium sulfate = magnesium + zinc sulfate. • determine the stoichiometric numbers. Construct the rate. Magnesium And Zinc Sulfate Balanced Equation.
From exowmpjlt.blob.core.windows.net
Zinc Sulfate + Magnesium Equation at Marcia Balser blog Magnesium And Zinc Sulfate Balanced Equation Derive chemical equations from narrative descriptions of chemical reactions. • assemble the rate term for. • balance the chemical equation. The balanced chemical equation for this reaction is: Write and balance chemical equations in molecular,. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: The chemical equation described in section 4.1 is balanced, meaning. Magnesium And Zinc Sulfate Balanced Equation.
From www.chegg.com
Solved 14.Which of the following is the properly balanced Magnesium And Zinc Sulfate Balanced Equation Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: Derive chemical equations from narrative descriptions of chemical reactions. Explain the roles of subscripts and coefficients in chemical equations. There are three main steps for. Magnesium And Zinc Sulfate Balanced Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Chemical For Zinc Sulfuric Magnesium And Zinc Sulfate Balanced Equation Balance a chemical equation when given the unbalanced equation. Zinc + magnesium sulfate = magnesium + zinc sulfate. Construct the rate of reaction expression for: There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: •. Magnesium And Zinc Sulfate Balanced Equation.
From www.youtube.com
How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric acid + Zinc) YouTube Magnesium And Zinc Sulfate Balanced Equation Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: Explain the roles of subscripts and coefficients in chemical equations. Zinc + magnesium sulfate = magnesium + zinc sulfate. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. There are three. Magnesium And Zinc Sulfate Balanced Equation.
From www.youtube.com
MgSO4+NaOH=Mg(OH)2+Na2SO4. balance the chemical equation mydocumentary838. YouTube Magnesium And Zinc Sulfate Balanced Equation Write and balance chemical equations in molecular,. • determine the stoichiometric numbers. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: Explain the roles of subscripts and coefficients in chemical equations. The balanced chemical equation for this reaction is: Balance a chemical equation when given the unbalanced equation. Zn + mgso4 = mg +. Magnesium And Zinc Sulfate Balanced Equation.
From hxenntpwz.blob.core.windows.net
Magnesium And Zinc Sulphate Equation at Mark Leary blog Magnesium And Zinc Sulfate Balanced Equation Write and balance chemical equations in molecular,. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Derive chemical equations from narrative descriptions of chemical reactions. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: Construct the rate of reaction expression for: Zinc +. Magnesium And Zinc Sulfate Balanced Equation.
From www.slideserve.com
PPT Net Ionic in SR PowerPoint Presentation, free download ID5979486 Magnesium And Zinc Sulfate Balanced Equation Construct the rate of reaction expression for: • assemble the rate term for. There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. • determine the stoichiometric numbers. Zinc + magnesium sulfate = magnesium + zinc sulfate. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution). Derive chemical. Magnesium And Zinc Sulfate Balanced Equation.
From www.toppr.com
Convert the following word equations into molecular equation and balance them. 1) Ammonium Magnesium And Zinc Sulfate Balanced Equation There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: Balance a chemical equation when given the unbalanced equation. Zn + mgso_4 mg + znso_4 plan: Derive chemical equations from narrative descriptions of chemical reactions. •. Magnesium And Zinc Sulfate Balanced Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Chemical For Zinc Sulfuric Magnesium And Zinc Sulfate Balanced Equation Construct the rate of reaction expression for: Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. The balanced chemical equation for this reaction is: • determine the stoichiometric numbers. The. Magnesium And Zinc Sulfate Balanced Equation.
From www.ashinthewild.com
Iron And Copper Sulfate Balanced Equation Ash in The Wild Magnesium And Zinc Sulfate Balanced Equation • balance the chemical equation. The balanced chemical equation for this reaction is: Balance a chemical equation when given the unbalanced equation. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: Zn + mgso_4 mg + znso_4 plan: Explain the roles of subscripts and coefficients in chemical equations. There are three main steps for. Magnesium And Zinc Sulfate Balanced Equation.
From www.slideshare.net
Replacement reactions Magnesium And Zinc Sulfate Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. • balance the chemical equation. Construct the rate of reaction expression for: Derive chemical equations from narrative descriptions of chemical reactions. There are three main steps for writing the net ionic equation for mg + znso4 =. Magnesium And Zinc Sulfate Balanced Equation.
From gioxcpnsw.blob.core.windows.net
Magnesium And Zinc Sulfate Net Ionic Equation at Steve Williamson blog Magnesium And Zinc Sulfate Balanced Equation Write and balance chemical equations in molecular,. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced chemical equation for this reaction is: • assemble the rate term for. Derive chemical equations from narrative descriptions. Magnesium And Zinc Sulfate Balanced Equation.
From www.nagwa.com
Question Video Identifying the Equation That Describes What Happens to Magnesium When Reacted Magnesium And Zinc Sulfate Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. • assemble the rate term for. Balance a chemical equation when given the unbalanced equation. Zn +. Magnesium And Zinc Sulfate Balanced Equation.
From www.toppr.com
Write a balanced chemical equation with the help of chemical symbols and formulas for the Magnesium And Zinc Sulfate Balanced Equation Zinc + magnesium sulfate = magnesium + zinc sulfate. Construct the rate of reaction expression for: Explain the roles of subscripts and coefficients in chemical equations. • assemble the rate term for. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Write and balance chemical equations. Magnesium And Zinc Sulfate Balanced Equation.
From hxenntpwz.blob.core.windows.net
Magnesium And Zinc Sulphate Equation at Mark Leary blog Magnesium And Zinc Sulfate Balanced Equation There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. • balance the chemical equation. Derive chemical equations from narrative descriptions of chemical reactions. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Zn + mgso_4 mg. Magnesium And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between sulfuric acid and zinc hydroride Magnesium And Zinc Sulfate Balanced Equation Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: The balanced chemical equation for this reaction is: Explain the roles of subscripts and coefficients in chemical equations. Zinc + magnesium sulfate = magnesium + zinc sulfate. • assemble the rate term for. Derive chemical equations from narrative descriptions of chemical reactions. • determine the. Magnesium And Zinc Sulfate Balanced Equation.
From w20.b2m.cz
Zn Mg No3 2 EDUCA Magnesium And Zinc Sulfate Balanced Equation Zn + mgso4 = mg + zn(so4) is a single displacement (substitution). Construct the rate of reaction expression for: The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. There are three main steps for writing the net ionic equation for mg + znso4 = zn +. Magnesium And Zinc Sulfate Balanced Equation.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Magnesium And Zinc Sulfate Balanced Equation Write and balance chemical equations in molecular,. Balance a chemical equation when given the unbalanced equation. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced chemical equation for this reaction is: Zn + mgso₄ → znso₄ + mg let's break down this equation step. Magnesium And Zinc Sulfate Balanced Equation.
From www.scibond.com
How to represent a chemical reaction? SciBond Magnesium And Zinc Sulfate Balanced Equation Derive chemical equations from narrative descriptions of chemical reactions. There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. • determine the stoichiometric numbers. Zn + mgso_4 mg + znso_4 plan: Write and balance chemical equations in molecular,. Zn + mgso₄ → znso₄ + mg let's break down this equation step. Magnesium And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced chemical equations 1 zinc react with sulphate and hydrogen Magnesium And Zinc Sulfate Balanced Equation • balance the chemical equation. Explain the roles of subscripts and coefficients in chemical equations. • determine the stoichiometric numbers. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. The balanced chemical equation for this reaction is: Zn + mgso₄ → znso₄ + mg let's break down this equation. Magnesium And Zinc Sulfate Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Mg + ZnSO4 = Zn + MgSO4 (See Note in Descp) YouTube Magnesium And Zinc Sulfate Balanced Equation • determine the stoichiometric numbers. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: • balance the chemical equation. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Limiting reagent can be computed for a balanced equation by entering the. Magnesium And Zinc Sulfate Balanced Equation.
From hxekwfnye.blob.core.windows.net
Magnesium + Zinc Sulphate Equation at Jimmy Walker blog Magnesium And Zinc Sulfate Balanced Equation Balance a chemical equation when given the unbalanced equation. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution). • determine the stoichiometric numbers. The balanced chemical equation for this reaction is: Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Explain the roles of subscripts and. Magnesium And Zinc Sulfate Balanced Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Reaction of Zinc with Magnesium And Zinc Sulfate Balanced Equation • determine the stoichiometric numbers. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Derive chemical equations from narrative descriptions of chemical reactions. • balance the chemical equation. Explain the roles of subscripts and coefficients in chemical equations. Zinc + magnesium sulfate = magnesium + zinc sulfate. Balance a. Magnesium And Zinc Sulfate Balanced Equation.
From exowmpjlt.blob.core.windows.net
Zinc Sulfate + Magnesium Equation at Marcia Balser blog Magnesium And Zinc Sulfate Balanced Equation Construct the rate of reaction expression for: Zn + mgso_4 mg + znso_4 plan: • determine the stoichiometric numbers. • balance the chemical equation. Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that. Magnesium And Zinc Sulfate Balanced Equation.
From www.youtube.com
How to Write the Formula for Magnesium sulfate YouTube Magnesium And Zinc Sulfate Balanced Equation • balance the chemical equation. The balanced chemical equation for this reaction is: There are three main steps for writing the net ionic equation for mg + znso4 = zn + mgso4. • determine the stoichiometric numbers. • assemble the rate term for. Write and balance chemical equations in molecular,. Zn + mgso4 = mg + zn(so4) is a single. Magnesium And Zinc Sulfate Balanced Equation.
From www.numerade.com
Using the activity series (Table 4.5), write balanced chemical equations for the following Magnesium And Zinc Sulfate Balanced Equation The balanced chemical equation for this reaction is: Zn + mgso₄ → znso₄ + mg let's break down this equation step by step: • balance the chemical equation. Zn + mgso_4 mg + znso_4 plan: Write and balance chemical equations in molecular,. • assemble the rate term for. The chemical equation described in section 4.1 is balanced, meaning that equal. Magnesium And Zinc Sulfate Balanced Equation.
From www.numerade.com
SOLVED REPORT SHEET LAB Chemical Reactions and Equations 10. Magnesium and Oxygen Initial Magnesium And Zinc Sulfate Balanced Equation Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. • balance the chemical equation. Derive chemical equations from narrative descriptions of chemical reactions. The balanced chemical equation for this reaction is: Zn + mgso_4 mg + znso_4 plan: • determine the stoichiometric numbers. Explain the roles of subscripts and. Magnesium And Zinc Sulfate Balanced Equation.
From chemisthunter.com
Zn + MgSO4 = Mg + ZnSO4 Magnesium And Zinc Sulfate Balanced Equation • assemble the rate term for. Zinc + magnesium sulfate = magnesium + zinc sulfate. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Derive chemical equations from narrative descriptions of chemical reactions. Explain the roles of subscripts and coefficients in chemical equations. Limiting reagent can. Magnesium And Zinc Sulfate Balanced Equation.
From brainly.in
What is the ionic equation for magnesium + zinc sulphate gives magnesium sulfate + zinc Brainly.in Magnesium And Zinc Sulfate Balanced Equation Explain the roles of subscripts and coefficients in chemical equations. Write and balance chemical equations in molecular,. Zn + mgso_4 mg + znso_4 plan: • determine the stoichiometric numbers. Zinc + magnesium sulfate = magnesium + zinc sulfate. Construct the rate of reaction expression for: • balance the chemical equation. Derive chemical equations from narrative descriptions of chemical reactions. •. Magnesium And Zinc Sulfate Balanced Equation.
From www.chegg.com
Solved Translate Each Word Equation into a Balanced Chemical Magnesium And Zinc Sulfate Balanced Equation Zn + mgso_4 mg + znso_4 plan: Construct the rate of reaction expression for: • assemble the rate term for. Explain the roles of subscripts and coefficients in chemical equations. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. The balanced chemical equation for this reaction. Magnesium And Zinc Sulfate Balanced Equation.
From exowmpjlt.blob.core.windows.net
Zinc Sulfate + Magnesium Equation at Marcia Balser blog Magnesium And Zinc Sulfate Balanced Equation Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical equations. Zn + mgso4 = mg + zn(so4) is a single displacement (substitution). Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. There are three main steps for writing the net. Magnesium And Zinc Sulfate Balanced Equation.