Baking Soda Reaction Equation . This chemistry video tutorial discusses the reaction between baking soda and vinegar. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. Balanced chemical equation for baking soda and vinegar reaction. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. It's also how you can.
from www.chegg.com
Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. This chemistry video tutorial discusses the reaction between baking soda and vinegar. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Balanced chemical equation for baking soda and vinegar reaction. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. It's also how you can. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one.
Solved What would be the proper mechanism of the reaction of
Baking Soda Reaction Equation The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Balanced chemical equation for baking soda and vinegar reaction. It's also how you can. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. This chemistry video tutorial discusses the reaction between baking soda and vinegar. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate.
From www.pinterest.com
Vinegar Baking Soda Demonstrations Middle school chemistry, Middle Baking Soda Reaction Equation In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Balanced chemical equation for baking soda and vinegar reaction. It's also how you can. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate.. Baking Soda Reaction Equation.
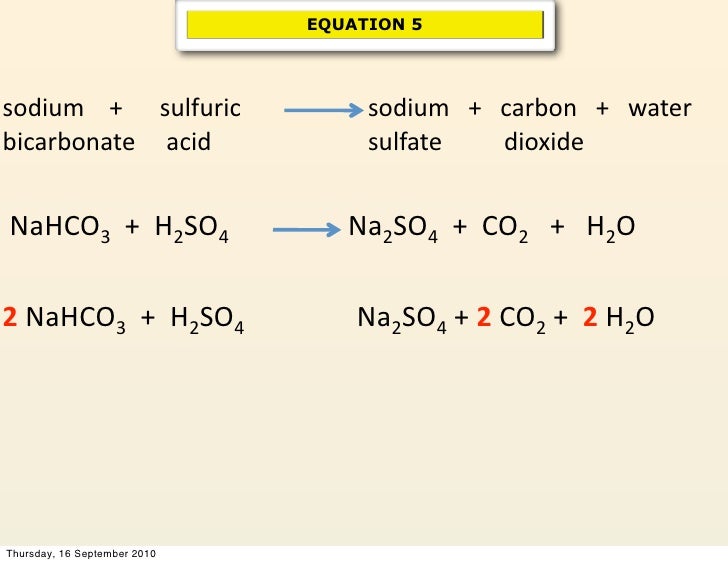
From classmediacambridge.z5.web.core.windows.net
Chemistry Baking Soda And Vinegar Baking Soda Reaction Equation Balanced chemical equation for baking soda and vinegar reaction. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. This chemistry video tutorial discusses the reaction. Baking Soda Reaction Equation.
From readingandwritingprojectcom.web.fc2.com
baking soda and vinegar reaction equation Baking Soda Reaction Equation Balanced chemical equation for baking soda and vinegar reaction. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. In this lab, students will examine the chemical reaction between baking soda and vinegar, and. Baking Soda Reaction Equation.
From team-cartwright.com
Fizzy Heart Science Experiment Team Cartwright Baking Soda Reaction Equation In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Balanced chemical equation for baking soda and vinegar reaction. This chemistry video tutorial discusses the reaction between baking soda and vinegar. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. Sodium bicarbonate, also known. Baking Soda Reaction Equation.
From klaxiajzu.blob.core.windows.net
Baking Soda And Vinegar Reaction Chemical Formula at Genevie Barraza blog Baking Soda Reaction Equation In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate.. Baking Soda Reaction Equation.
From www.teachoo.com
Case Based MCQ Sanjana while preparing cake used Science Class 10 Baking Soda Reaction Equation It's also how you can. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. A sodium cation (na+) and a bicarbonate anion. Baking Soda Reaction Equation.
From www.youtube.com
Baking Soda and Vinegar Equation and Reaction YouTube Baking Soda Reaction Equation A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. This chemistry video tutorial discusses. Baking Soda Reaction Equation.
From www.teachoo.com
Case Based MCQ Sanjana while preparing cake used Science Class 10 Baking Soda Reaction Equation In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to. Baking Soda Reaction Equation.
From logankruwjacobs.blogspot.com
Baking Soda and Vinegar Reaction LogankruwJacobs Baking Soda Reaction Equation Balanced chemical equation for baking soda and vinegar reaction. This chemistry video tutorial discusses the reaction between baking soda and vinegar. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. It's also how you can. In this lab, students will examine the chemical reaction. Baking Soda Reaction Equation.
From brainly.in
How washing soda is obtained from baking soda? give a balanced chemical Baking Soda Reaction Equation Balanced chemical equation for baking soda and vinegar reaction. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. Sodium bicarbonate, also known as baking soda or bicarbonate of. Baking Soda Reaction Equation.
From www.chegg.com
Solved What would be the proper mechanism of the reaction of Baking Soda Reaction Equation This chemistry video tutorial discusses the reaction between baking soda and vinegar. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. Balanced chemical equation for baking soda and vinegar reaction. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. Baking soda and vinegar react to. Baking Soda Reaction Equation.
From lr.leskanaris.com
Equation for Reaction Between Baking Soda and Vinegar Interesting 2024 Baking Soda Reaction Equation In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. It's also how you can. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is. Baking Soda Reaction Equation.
From sciencenotes.org
Chemical Equation for Baking Soda and Vinegar Reaction Baking Soda Reaction Equation In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Balanced chemical equation for baking soda and vinegar reaction. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. This chemistry video tutorial discusses the reaction between baking soda and vinegar. It's also how you. Baking Soda Reaction Equation.
From joistghol.blob.core.windows.net
Lime Water Vinegar Equation at Jim Whitten blog Baking Soda Reaction Equation The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. This chemistry video tutorial discusses the reaction between baking soda and vinegar. In. Baking Soda Reaction Equation.
From www.thoughtco.com
Baking Soda Molecular Formula Sodium Bicarbonate Baking Soda Reaction Equation Balanced chemical equation for baking soda and vinegar reaction. It's also how you can. This chemistry video tutorial discusses the reaction between baking soda and vinegar. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these. Baking Soda Reaction Equation.
From treatybottle13.pythonanywhere.com
Fun Baking Soda And Water Chemical Equation Solver Predict Products Baking Soda Reaction Equation It's also how you can. Balanced chemical equation for baking soda and vinegar reaction. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. The decomposition. Baking Soda Reaction Equation.
From shotprofessional22.gitlab.io
Supreme Baking Soda And Vinegar Word Equation C Formula Physics Baking Soda Reaction Equation Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. This chemistry video tutorial discusses the reaction between baking soda and vinegar. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. One mole of sodium. Baking Soda Reaction Equation.
From www.pinterest.com
A handson inquiry activity where students measure the amount of carbon Baking Soda Reaction Equation Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. Sodium bicarbonate, also known. Baking Soda Reaction Equation.
From www.apartmenttherapy.com
Don't Mix Baking Soda and Vinegar for Cleaning Apartment Therapy Baking Soda Reaction Equation Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. This chemistry video tutorial discusses the reaction between baking soda and vinegar. In. Baking Soda Reaction Equation.
From dxooumcca.blob.core.windows.net
Baking Soda And Water Reaction Formula at Monique Perry blog Baking Soda Reaction Equation It's also how you can. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. A sodium cation (na+) and a bicarbonate. Baking Soda Reaction Equation.
From klagnzxga.blob.core.windows.net
Baking Powder And Vinegar Chemical Reaction at James Bloomer blog Baking Soda Reaction Equation This chemistry video tutorial discusses the reaction between baking soda and vinegar. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. A sodium. Baking Soda Reaction Equation.
From loecjbvmr.blob.core.windows.net
Baking Soda + Water Equation at Mabel Robinson blog Baking Soda Reaction Equation One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. It's also how you can. Balanced chemical equation for baking soda and vinegar reaction. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Sodium bicarbonate, also known as baking. Baking Soda Reaction Equation.
From ceznlwqf.blob.core.windows.net
Vinegar And Baking Soda Reaction Safe at Harold Lucey blog Baking Soda Reaction Equation Balanced chemical equation for baking soda and vinegar reaction. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts. Baking Soda Reaction Equation.
From www.coursehero.com
[Solved] 6. The equation for the reaction between baking soda and Baking Soda Reaction Equation The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic. Baking Soda Reaction Equation.
From www.alamy.com
Baking Soda and VinegarBalloon ExperimentScience Stock Vector Image Baking Soda Reaction Equation Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. Balanced chemical equation for baking soda and vinegar reaction. It's also how you can. This chemistry video tutorial discusses the reaction between baking soda and vinegar. One mole of sodium bicarbonate (baking soda) reacts with. Baking Soda Reaction Equation.
From www.tessshebaylo.com
Chemical Equation For Baking Soda Citric Acid And Water Tessshebaylo Baking Soda Reaction Equation Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. Balanced chemical equation for baking soda and vinegar reaction. It's also how you can. Baking soda and vinegar react to neutralise. Baking Soda Reaction Equation.
From dxonidvhd.blob.core.windows.net
Baking Soda Ka Formula Kya Hai at Dorothy Martinez blog Baking Soda Reaction Equation Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. It's also how you can. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked. Baking Soda Reaction Equation.
From lifeovercs.com
Baking Soda and Vinegar Preschoolers Science Experiment Life Over C's Baking Soda Reaction Equation The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. In this lab, students will examine the chemical reaction between baking soda and vinegar, and. Baking Soda Reaction Equation.
From klauulrxu.blob.core.windows.net
What Is A Baking Soda Reaction at Teresa Ranallo blog Baking Soda Reaction Equation It's also how you can. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one. Balanced chemical equation for baking soda and vinegar reaction. This chemistry. Baking Soda Reaction Equation.
From www.youtube.com
Chemical reaction mixing baking soda and vinegar YouTube Baking Soda Reaction Equation Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. It's also how you can. Sodium bicarbonate, also known as baking soda or bicarbonate of soda,. Baking Soda Reaction Equation.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH Baking Soda Reaction Equation Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. Balanced chemical equation for baking soda and vinegar reaction. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. This chemistry video tutorial. Baking Soda Reaction Equation.
From www.vernier.com
Vinegar and Baking Soda Reaction Heat Up or Cool Down? Vernier Baking Soda Reaction Equation The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. This chemistry video tutorial discusses the reaction between baking soda and vinegar. Baking soda and vinegar react to neutralise each other ( vinegar is an acid and baking soda an alkali ) releasing carbon dioxide. One mole of. Baking Soda Reaction Equation.
From shotprofessional22.gitlab.io
Ideal What Is The Balanced Chemical Equation For Vinegar And Baking Baking Soda Reaction Equation In this lab, students will examine the chemical reaction between baking soda and vinegar, and mix different amounts of these household. Balanced chemical equation for baking soda and vinegar reaction. It's also how you can. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. This chemistry video. Baking Soda Reaction Equation.
From www.expii.com
What Is a Chemical Reaction? — Overview & Examples Expii Baking Soda Reaction Equation Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is a chemical compound with the formula nahco3 and the iupac designation sodium hydrogencarbonate. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. It's also how you can. A sodium cation (na+) and a bicarbonate anion. Baking Soda Reaction Equation.
From signalticket9.pythonanywhere.com
Matchless Baking Soda Balanced Equation Butane Oxygen Reaction Baking Soda Reaction Equation A sodium cation (na+) and a bicarbonate anion (hco3) combine to form this salt. This chemistry video tutorial discusses the reaction between baking soda and vinegar. The decomposition reaction of sodium bicarbonate or baking soda is an important chemical reaction for baking because it helps baked goods rise. Sodium bicarbonate, also known as baking soda or bicarbonate of soda, is. Baking Soda Reaction Equation.