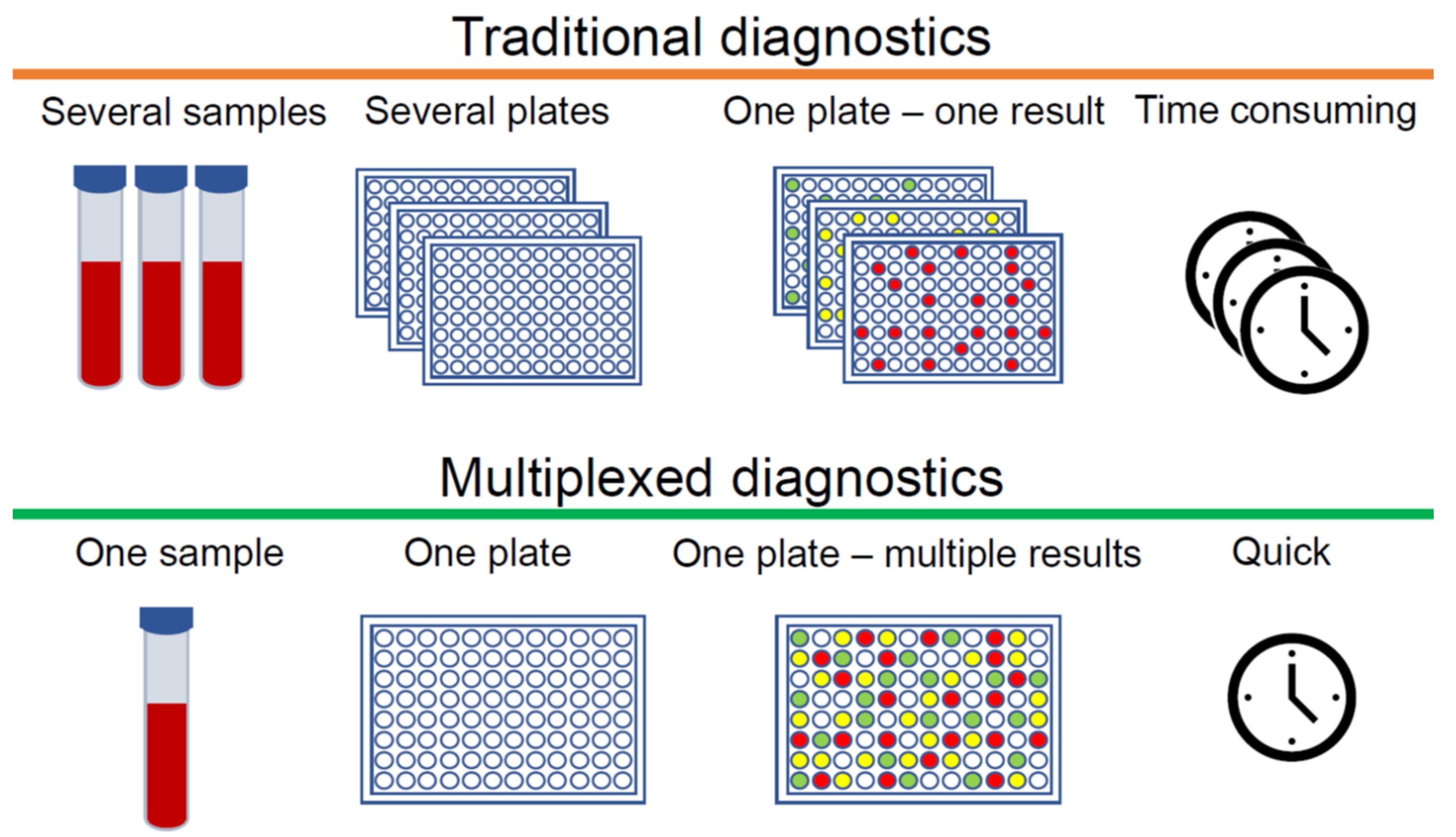
Single Analyte Test Example . To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” Define single analyte test and profile test, and give example of each. What is test method validation? This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. For example, you may have serum glucose tested on three platforms: A single module brand x instrument and a different brand x model that contains two. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use.
from www.mdpi.com
This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. What is test method validation? “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. A single module brand x instrument and a different brand x model that contains two. For example, you may have serum glucose tested on three platforms: To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. Define single analyte test and profile test, and give example of each.
Sensors Free FullText Multiplexed Nanobiosensors Current Trends
Single Analyte Test Example Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. For example, you may have serum glucose tested on three platforms: What is test method validation? To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” Define single analyte test and profile test, and give example of each. A single module brand x instrument and a different brand x model that contains two.
From www.researchgate.net
Test characteristics for analytes, a repeatability, and... Download Single Analyte Test Example A single module brand x instrument and a different brand x model that contains two. Define single analyte test and profile test, and give example of each. For example, you may have serum glucose tested on three platforms: What is test method validation? “as per fda method validation is defined as the process of proving (through scientific studies) that an. Single Analyte Test Example.
From www.thermofisher.cn
Protein Profiling with High Plex ProcartaPlex Assays Thermo Fisher Single Analyte Test Example To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. What is test method validation? This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended. Single Analyte Test Example.
From www.jacksonimmuno.com
Lateral Flow Immunoassays Jackson ImmunoResearch Single Analyte Test Example “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” For example, you may have serum glucose tested on three platforms: To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. This chapter provides theoretical and practical information in determining the. Single Analyte Test Example.
From nsilabsolutions.com
Cyanide 125mL IC019 NSI Lab Solutions Single Analyte Test Example “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” Define single analyte test and profile test, and give example of each. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended. Single Analyte Test Example.
From www.researchgate.net
Singleanalyte measurements using microengraving. (a) Measurement of Single Analyte Test Example A single module brand x instrument and a different brand x model that contains two. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. What is test method validation? Define single analyte test and profile test, and give example of each. For example, you may. Single Analyte Test Example.
From www.metabolon.com
Custom Single Analyte Assay Metabolon Single Analyte Test Example A single module brand x instrument and a different brand x model that contains two. This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. Define single analyte test and profile test, and give example of each. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. What. Single Analyte Test Example.
From nicoyalife.com
4 Types of Binding Assays you can do with SPR OpenSPR Nicoya Single Analyte Test Example For example, you may have serum glucose tested on three platforms: Define single analyte test and profile test, and give example of each. What is test method validation? This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable.. Single Analyte Test Example.
From www.slideserve.com
PPT Tests of Significance PowerPoint Presentation, free download ID Single Analyte Test Example What is test method validation? Define single analyte test and profile test, and give example of each. “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample.. Single Analyte Test Example.
From www.researchgate.net
a) Typical response profile of graphene‐based gas sensor toward 100 ppb Single Analyte Test Example Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. A single module brand x instrument and a different brand x model that contains two. For example, you may have serum glucose tested on three platforms: Define single analyte test and profile test, and give example. Single Analyte Test Example.
From www.researchgate.net
(a) Schematic representation of a surfaceconjugationbased MPS Single Analyte Test Example A single module brand x instrument and a different brand x model that contains two. What is test method validation? “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” For example, you may have serum glucose tested on three platforms: Define single analyte test. Single Analyte Test Example.
From www.chemetrics.com
Ozone Single Analyte Machine (SAM) Kit CHEMetrics Single Analyte Test Example A single module brand x instrument and a different brand x model that contains two. For example, you may have serum glucose tested on three platforms: Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. This chapter provides theoretical and practical information in determining the. Single Analyte Test Example.
From slideplayer.com
Volume 45, Issue 4, Pages (October 2016) ppt download Single Analyte Test Example “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. To ensure correct diagnosis and treatment, clinical laboratory testing must be. Single Analyte Test Example.
From www.researchgate.net
a LFIA before analyses with two analytes in the sample drop, the Single Analyte Test Example What is test method validation? To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. For example, you may have serum glucose tested on three platforms: “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” This chapter provides theoretical and. Single Analyte Test Example.
From pubs.rsc.org
A new pointofcare test for the diagnosis of infectious diseases based Single Analyte Test Example This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. A single module brand x instrument and a different brand x model that contains two. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. To ensure correct. Single Analyte Test Example.
From www.researchgate.net
A shows the composition of the polar analyte library screened for test Single Analyte Test Example To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. For example, you may have serum glucose tested on three platforms: A single module brand x instrument and a different brand x model that contains two. “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is. Single Analyte Test Example.
From www.researchgate.net
Analyte confirmation by ion intensity ratio comparison of dexamethasone Single Analyte Test Example This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. “as per fda method. Single Analyte Test Example.
From www.indiamart.com
Nutritional Non GLP GC FID/ECD Single Analyte method Service, in Single Analyte Test Example What is test method validation? For example, you may have serum glucose tested on three platforms: “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. To. Single Analyte Test Example.
From www.medlabmag.com
Technical Comparison of Immunoassay and Mass Spectrometry June 2016 Single Analyte Test Example Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. For example, you may have serum glucose tested on three platforms: “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.”. Single Analyte Test Example.
From www.youtube.com
Introduction to Chromatography 6 Retention Time YouTube Single Analyte Test Example Define single analyte test and profile test, and give example of each. This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. A single module brand x instrument. Single Analyte Test Example.
From www.researchgate.net
(PDF) A multianalyte PCR blood test outperforms single analyte ELISAs Single Analyte Test Example A single module brand x instrument and a different brand x model that contains two. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. What is test method validation? “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” For. Single Analyte Test Example.
From www.researchgate.net
Calculation of optimal minimum required dilution (MRD) results of Single Analyte Test Example A single module brand x instrument and a different brand x model that contains two. For example, you may have serum glucose tested on three platforms: To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. What is. Single Analyte Test Example.
From www.chegg.com
Solved TEST 111 MORSE TYPE Choose all possible answers. Ex. Single Analyte Test Example What is test method validation? Define single analyte test and profile test, and give example of each. This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. For. Single Analyte Test Example.
From nicoyalife.com
MCK vs SCK Two common methods for analysis with SPR Nicoya Single Analyte Test Example For example, you may have serum glucose tested on three platforms: A single module brand x instrument and a different brand x model that contains two. “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” Define single analyte test and profile test, and give. Single Analyte Test Example.
From www.aging-us.com
Prediction of chronological and biological age from laboratory data Aging Single Analyte Test Example Define single analyte test and profile test, and give example of each. “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” A single module brand x instrument and a different brand x model that contains two. Analytical method validation is the process used to. Single Analyte Test Example.
From www.mdpi.com
Sensors Free FullText Multiplexed Nanobiosensors Current Trends Single Analyte Test Example This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” What is test method validation? A single module brand x instrument and a different brand x model. Single Analyte Test Example.
From exovevmgr.blob.core.windows.net
Titration Lab Quiz at Roger Owen blog Single Analyte Test Example “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” For example, you may have serum glucose tested on three platforms: Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use.. Single Analyte Test Example.
From www.slideserve.com
PPT Immunochemical Methods and Biosensors for pollutants Single Analyte Test Example A single module brand x instrument and a different brand x model that contains two. What is test method validation? This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its. Single Analyte Test Example.
From www.chemetrics.com
Nitrite in Water Analysis New NED Method Tests Available Single Analyte Test Example To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” A single module brand x instrument and a different brand x model that contains two. This chapter provides theoretical and practical. Single Analyte Test Example.
From www.researchgate.net
(A) Fluorescence response pattern ((I − I 0 )/I 0 ) obtained by PDIs Single Analyte Test Example “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” What is test method validation? To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. A single module brand x instrument and a different brand x model that contains two. For. Single Analyte Test Example.
From www.researchgate.net
Types of tumor biomarker tests. The figure conceptually displays a Single Analyte Test Example For example, you may have serum glucose tested on three platforms: This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. A single module brand x instrument and a different brand x model that contains two. Analytical method validation is the process used to authenticate that the analytical procedure employed for a. Single Analyte Test Example.
From www.researchgate.net
Performance metrics for the vs the single analyte ELISAs Single Analyte Test Example Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. For example, you may have serum glucose tested on three platforms: To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. Define single analyte test and profile test, and give example of. Single Analyte Test Example.
From nsilabsolutions.com
Nitrite 125mL IC031 NSI Lab Solutions Single Analyte Test Example “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” Define single analyte test and profile test, and give example of each. What is test method validation? For example, you may have serum glucose tested on three platforms: This chapter provides theoretical and practical information. Single Analyte Test Example.
From www.technologynetworks.com
A multianalyte algorithm PCRbased blood test outperforms single Single Analyte Test Example To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. Define single analyte test and profile test, and give example of each. What is test method validation? A single module brand x instrument and a different brand x. Single Analyte Test Example.
From exolqsiut.blob.core.windows.net
Titration Error Nedir at Elva Gurney blog Single Analyte Test Example Analytical method validation is the process used to authenticate that the analytical procedure employed for a specific test is suitable for its intended use. To ensure correct diagnosis and treatment, clinical laboratory testing must be accurate and reliable. Define single analyte test and profile test, and give example of each. A single module brand x instrument and a different brand. Single Analyte Test Example.
From medunion.en.made-in-china.com
Home Use Clinics Hemoglobin Meter Monitoring System Hemoglobin Analyzer Single Analyte Test Example “as per fda method validation is defined as the process of proving (through scientific studies) that an analytical method is acceptable for its intended use.” A single module brand x instrument and a different brand x model that contains two. This chapter provides theoretical and practical information in determining the concentration of a single analyte in a sample. What is. Single Analyte Test Example.