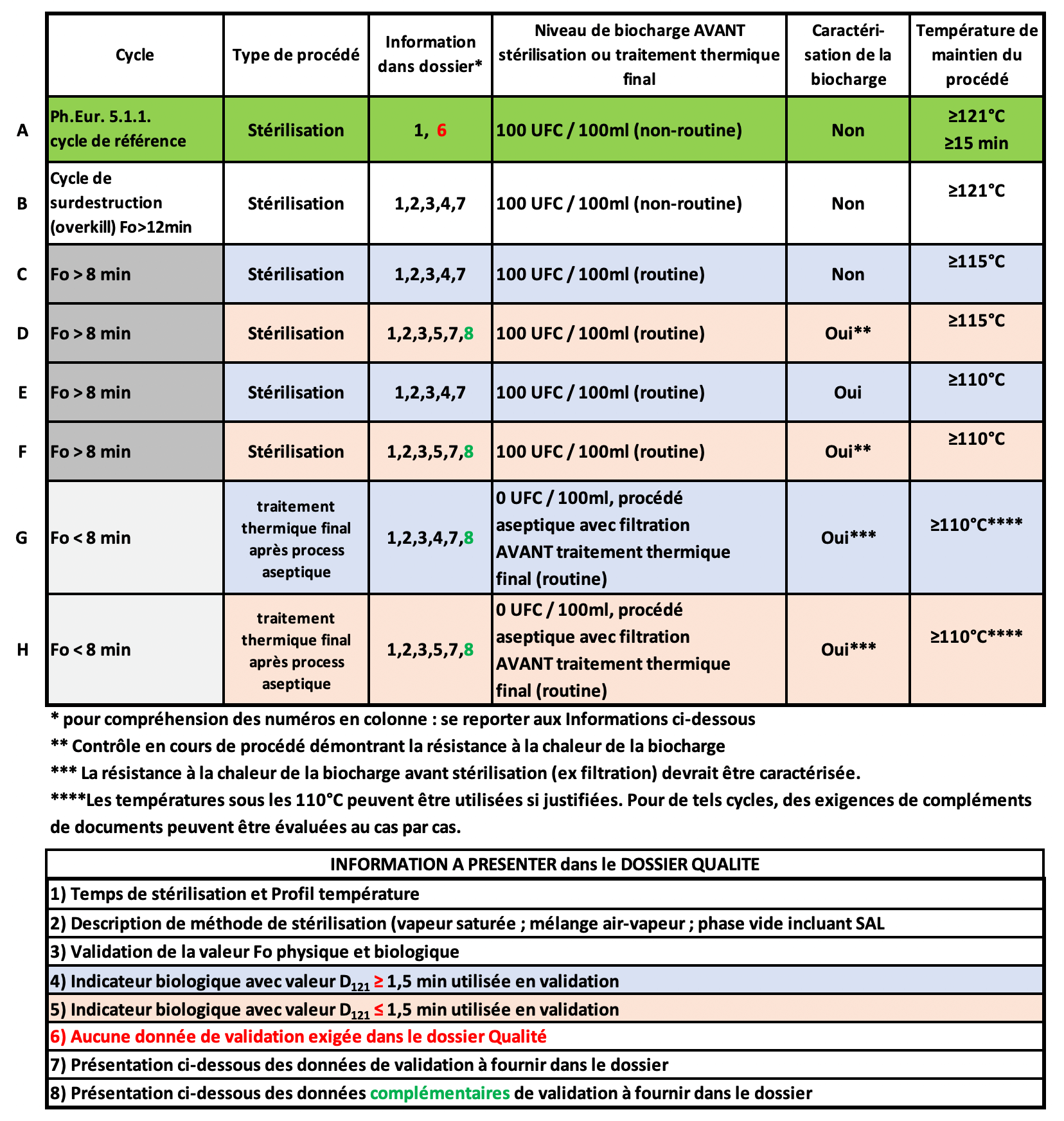
Sterilization Guidelines Ema . guideline on sterilisation of the medicinal product, active substance, excipient and primary container. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. Discusses approaches to sterilization of products, equipment and packaging. and terminal sterilization processes.
from www.a3p.org
the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. and terminal sterilization processes. ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. Discusses approaches to sterilization of products, equipment and packaging. guideline on sterilisation of the medicinal product, active substance, excipient and primary container.
New EMA Sterilization guideline GIC A3P Sterilization
Sterilization Guidelines Ema the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. Discusses approaches to sterilization of products, equipment and packaging. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. and terminal sterilization processes. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for.
From mavink.com
Eto Sterilization Process Flow Chart Sterilization Guidelines Ema ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. the purpose of this document is to provide guidance. Sterilization Guidelines Ema.
From present5.com
Cleaning Disinfection and Sterilization Meeting the CDC Guideline Sterilization Guidelines Ema Discusses approaches to sterilization of products, equipment and packaging. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. and terminal sterilization processes. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for. Sterilization Guidelines Ema.
From dokumen.tips
(PDF) Disinfection and sterilization guidelines what you need to know Sterilization Guidelines Ema guideline on sterilisation of the medicinal product, active substance, excipient and primary container. and terminal sterilization processes. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. Discusses approaches to sterilization of products, equipment and packaging. ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. guideline on the sterilisation of. Sterilization Guidelines Ema.
From dokumen.tips
(PDF) Sterilization Guidelines · STERILIZATION GUIDELINES 5 1. TERMS Sterilization Guidelines Ema Discusses approaches to sterilization of products, equipment and packaging. ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. the document. Sterilization Guidelines Ema.
From mediwish.com
Class 6 Indicators for Sterilization Explained Sterilization Guidelines Ema guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. and terminal sterilization processes. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. ema's new draft guideline entitled guideline on the. Sterilization Guidelines Ema.
From www.slideserve.com
PPT Disinfection and Sterilization Guidelines What You Need to Know Sterilization Guidelines Ema guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. guideline on sterilisation of the medicinal product, active substance, excipient and primary. Sterilization Guidelines Ema.
From www.researchgate.net
EMA decision tree for sterilization choices for aqueous products. EMA Sterilization Guidelines Ema guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile. Sterilization Guidelines Ema.
From www.a3p.org
New EMA Sterilization guideline GIC A3P Sterilization Sterilization Guidelines Ema ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. guidance. Sterilization Guidelines Ema.
From www.scribd.com
Sterilization Decision Tree EMA PDF Sterilization (Microbiology Sterilization Guidelines Ema this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. ema's new draft guideline entitled guideline on. Sterilization Guidelines Ema.
From array.aami.org
Medical Device Sterilization Modality Selection Decision Process Sterilization Guidelines Ema ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. Discusses approaches to sterilization of products, equipment and packaging. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. guidance is provided on the. Sterilization Guidelines Ema.
From www.pinterest.com
AAMI Sterilization Cycle Monitoring Sterilization Guidelines Ema Discusses approaches to sterilization of products, equipment and packaging. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. the. Sterilization Guidelines Ema.
From www.pinterest.com
Disinfection & Sterilization Guidelines Guidelines Library Sterilization Guidelines Ema and terminal sterilization processes. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. the purpose of this document is to provide guidance. Sterilization Guidelines Ema.
From www.buddingforensicexpert.in
Difference between Sterilisation and Disinfection Budding Forensic Expert Sterilization Guidelines Ema the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. Discusses approaches to sterilization of products, equipment and packaging. ema's new draft guideline entitled guideline on. Sterilization Guidelines Ema.
From www.a3p.org
New EMA Sterilization guideline GIC A3P Sterilization Sterilization Guidelines Ema guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. Discusses approaches to sterilization of products, equipment and packaging. and terminal sterilization processes. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. guideline on. Sterilization Guidelines Ema.
From wfhss-guidelines.com
Sterilization Wfhss Guidelines Sterilization Guidelines Ema Discusses approaches to sterilization of products, equipment and packaging. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. and terminal sterilization processes. guidance is provided on the documentation expected for sterile finished products, sterile. Sterilization Guidelines Ema.
From wfhss-guidelines.com
Sterilization Wfhss Guidelines Sterilization Guidelines Ema the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. ema's new draft guideline entitled guideline on the sterilisation of the medicinal product,. Sterilization Guidelines Ema.
From www.slideserve.com
PPT Disinfection and Sterilization Guidelines What You Need to Know Sterilization Guidelines Ema guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. and terminal sterilization processes. the purpose of this document is to provide guidance on. Sterilization Guidelines Ema.
From ask.pharmaguideline.com
Sterilization temperature Pharmaguideline Forum Sterilization Guidelines Ema this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. Discusses approaches to sterilization of products, equipment and. Sterilization Guidelines Ema.
From exokuwuse.blob.core.windows.net
Cms Sterilization Guidelines at Betty Gable blog Sterilization Guidelines Ema guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,.. Sterilization Guidelines Ema.
From www.linkedin.com
EMA Annex 1 of the guidelines on Good Manufacturing Practice Comments Sterilization Guidelines Ema the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. Discusses approaches to sterilization of products, equipment and packaging. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. . Sterilization Guidelines Ema.
From www.scribd.com
Guidelines For The Cleaning and Sterilization Of.14 PDF Surgery Sterilization Guidelines Ema Discusses approaches to sterilization of products, equipment and packaging. and terminal sterilization processes. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guideline on the sterilisation of the medicinal product, active. Sterilization Guidelines Ema.
From medicaldeviceacademy.com
How to select and help validate the best sterilization method? Sterilization Guidelines Ema guideline on sterilisation of the medicinal product, active substance, excipient and primary container. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. ema's new draft guideline entitled guideline on the sterilisation of the medicinal. Sterilization Guidelines Ema.
From www.yumpu.com
EMA Guideline on inuse s Sterilization Guidelines Ema the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. guideline on the sterilisation of the medicinal product, active substance, excipient and. Sterilization Guidelines Ema.
From www.scribd.com
Sterilization Guidelines 2010 PDF Verification And Validation Sterilization Guidelines Ema Discusses approaches to sterilization of products, equipment and packaging. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. . Sterilization Guidelines Ema.
From www.cambridge.org
Guideline for Disinfection and Sterilization of PrionContaminated Sterilization Guidelines Ema guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. and terminal sterilization processes. this guideline provides guidance on the documentation expected for sterile products. Sterilization Guidelines Ema.
From operonstrategist.com
FDA's Updated Sterilization Guidelines for Medical Device Operon Sterilization Guidelines Ema ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. this guideline provides guidance on the documentation expected for sterile products. Sterilization Guidelines Ema.
From www.scribd.com
New EMA Draft Guideline On Sterilisation PDF Sterilization Sterilization Guidelines Ema ema's new draft guideline entitled guideline on the sterilisation of the medicinal product, active substance,. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. and terminal sterilization processes. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the. Sterilization Guidelines Ema.
From www.slideserve.com
PPT Disinfection and Sterilization Guidelines What You Need to Know Sterilization Guidelines Ema guideline on sterilisation of the medicinal product, active substance, excipient and primary container. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. guidance. Sterilization Guidelines Ema.
From www.flinnsci.com
Sterilization Guidelines Flinn Scientific Sterilization Guidelines Ema guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. Discusses approaches to sterilization of products, equipment and packaging. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. and terminal sterilization processes. this guideline provides guidance on the. Sterilization Guidelines Ema.
From dxolyvvhu.blob.core.windows.net
Fda Sterilization Guidance at Erma Ferrel blog Sterilization Guidelines Ema guideline on sterilisation of the medicinal product, active substance, excipient and primary container. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. and terminal sterilization processes. Discusses approaches to sterilization of products, equipment and packaging. the purpose of. Sterilization Guidelines Ema.
From www.eurofins.com.au
Sterility Testing for sterile pharmaceutical products and medical Sterilization Guidelines Ema this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guideline on sterilisation of the medicinal product, active substance, excipient and primary. Sterilization Guidelines Ema.
From www.slideserve.com
PPT Disinfection and Sterilization Guidelines What You Need to Know Sterilization Guidelines Ema and terminal sterilization processes. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. Discusses approaches to sterilization of products, equipment. Sterilization Guidelines Ema.
From www.researchgate.net
EMA decision tree for sterilization choices for aqueous products. EMA Sterilization Guidelines Ema guideline on sterilisation of the medicinal product, active substance, excipient and primary container. Discusses approaches to sterilization of products, equipment and packaging. and terminal sterilization processes. this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. guidance is provided on the documentation expected for sterile finished products, sterile. Sterilization Guidelines Ema.
From www.hu-friedy.com
Follow These Critical Guidelines for Proper Sterilization HuFriedy Sterilization Guidelines Ema this guideline provides guidance on the documentation expected for sterile products in the quality dossier for a marketing. Discusses approaches to sterilization of products, equipment and packaging. and terminal sterilization processes. the document ema/chmp/cvmp/qwp/850374/2015 guideline on the sterilisation of the. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods. Sterilization Guidelines Ema.
From www.pharmoutsourcing.com
Parenteral Preparations, Challenges in Formulations Pharmaceutical Sterilization Guidelines Ema guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. the purpose of this document is to provide guidance on the selection of appropriate sterilization methods for. guideline on sterilisation of the medicinal product, active substance, excipient and primary container. this guideline provides guidance on the documentation expected for sterile products in. Sterilization Guidelines Ema.