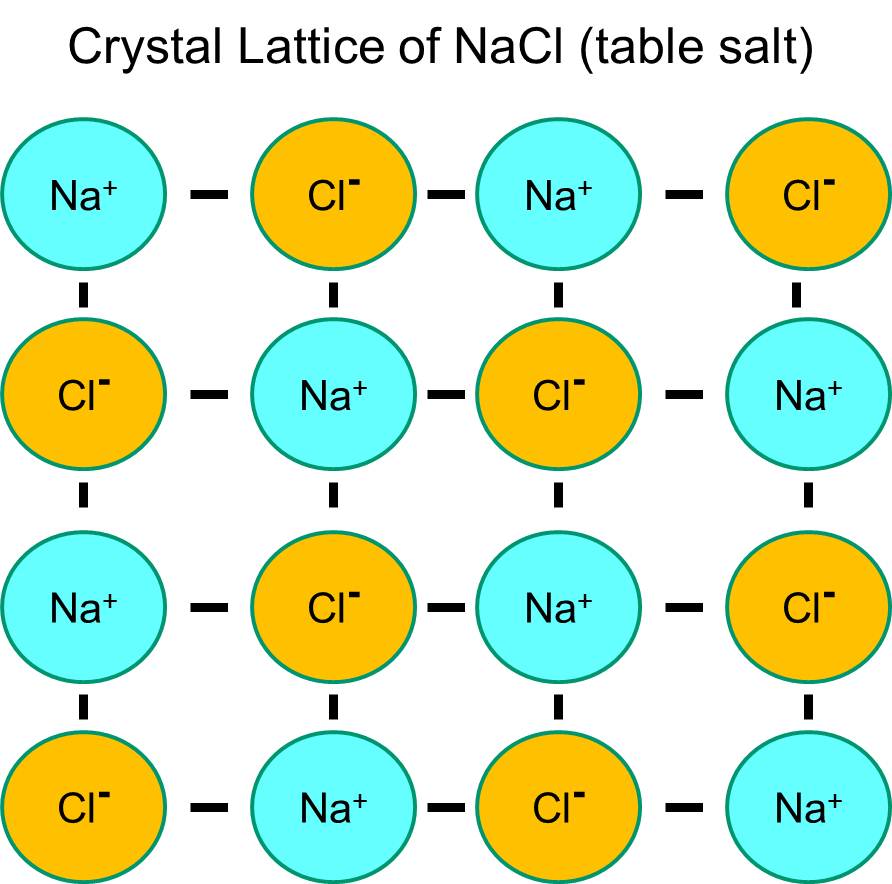
What Are Salt Bonds . A salt molecule contains atoms of two elements: Ionic bonding is the type of bonding that holds salts together. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. In the case of table salt, the positively charged. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,.
from sphweb.bumc.bu.edu
Ionic bonding is the type of bonding that holds salts together. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. A salt molecule contains atoms of two elements: In the case of table salt, the positively charged. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a.
Chemical Elements Atoms
What Are Salt Bonds To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. In the case of table salt, the positively charged. Ionic bonding is the type of bonding that holds salts together. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. A salt molecule contains atoms of two elements:
From www.researchgate.net
(a) Structure of complex salt III and hydrogen bonds between the What Are Salt Bonds Ionic bonding is the type of bonding that holds salts together. In the case of table salt, the positively charged. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. To better. What Are Salt Bonds.
From www.istockphoto.com
Vector Ballandstick Model Of Chemical Substance Icon Of Sodium Chloride What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. A salt molecule contains atoms of two elements: Salts. What Are Salt Bonds.
From www.snexplores.org
Explainer What are chemical bonds? What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. Salts are held together by ionic bonds, which are. What Are Salt Bonds.
From www.shutterstock.com
Salt molecule 2 443 images, photos et images vectorielles de stock What Are Salt Bonds In the case of table salt, the positively charged. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. Salts are held together by ionic bonds, which are formed when. What Are Salt Bonds.
From stock.adobe.com
Sodium chloride (NaCl) molecule structure in 3d vector illustration What Are Salt Bonds These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. In the case of table salt, the positively charged. To better understand why and how ions —. What Are Salt Bonds.
From en.wikipedia.org
Ionic bonding Wikipedia What Are Salt Bonds Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. Ionic bonding is the type of bonding that holds salts together. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. To better understand why and how ions — atoms that have. What Are Salt Bonds.
From www.architecturalecologies.cca.edu
Salt Bonds — Architectural Ecologies Lab What Are Salt Bonds In the case of table salt, the positively charged. Ionic bonding is the type of bonding that holds salts together. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. To better understand why and how ions — atoms that have a charge. What Are Salt Bonds.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID3118285 What Are Salt Bonds In the case of table salt, the positively charged. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. Ionic bonding is the type of bonding that holds salts together.. What Are Salt Bonds.
From www.architecturalecologies.cca.edu
Salt Bonds — Architectural Ecologies Lab What Are Salt Bonds These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. A salt molecule contains atoms of two elements: The bonds in salt compounds are called ionic because they both have. What Are Salt Bonds.
From sphweb.bumc.bu.edu
Chemical Elements Atoms What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. Ionic bonding is the type of bonding that holds salts together. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion.. What Are Salt Bonds.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. In the case of table salt, the positively charged. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,.. What Are Salt Bonds.
From sphweb.bumc.bu.edu
Chemical Elements Atoms What Are Salt Bonds A salt molecule contains atoms of two elements: In the case of table salt, the positively charged. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. The bonds in salt compounds. What Are Salt Bonds.
From ko.depositphotos.com
염화나트륨의 Nacl Model Vector Illustration Chemistry Model Salt Molecue What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. In the case of table salt, the positively charged. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. These atoms. What Are Salt Bonds.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID3118285 What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. A salt molecule contains atoms of two elements: Ionic bonding is the type of bonding that holds salts together. In the case of table salt, the positively charged. Salts are held together by. What Are Salt Bonds.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector What Are Salt Bonds These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. In the case of table salt, the positively charged. A salt molecule contains atoms of two elements: Ionic bonding is the type of bonding that holds salts together. Salts are held together by ionic bonds, which are formed when a positively charged ion is. What Are Salt Bonds.
From www.researchgate.net
Hydrogen bonds and salt bridges at the A1 body bottom. Of all shown What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. A salt molecule contains atoms of two elements: Salts. What Are Salt Bonds.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID7054809 What Are Salt Bonds In the case of table salt, the positively charged. Ionic bonding is the type of bonding that holds salts together. A salt molecule contains atoms of two elements: Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. These atoms arrange themselves into tidy cubes, with each sodium. What Are Salt Bonds.
From www.pikpng.com
Download Png Royalty Free Drawing Atoms Sodium Covalent Bonding Of What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. A salt molecule contains atoms of two elements: In the case of table salt, the positively charged. Salts are held together by ionic bonds, which are formed when a positively charged ion is. What Are Salt Bonds.
From thelatest.modere.com
Why Science Says You Need BondBuilding Hair Care The Latest What Are Salt Bonds In the case of table salt, the positively charged. A salt molecule contains atoms of two elements: The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. Ionic bonding is the type of bonding that holds salts together. These atoms arrange themselves into. What Are Salt Bonds.
From thelatest.modere.com.au
WHY SCIENCE SAYS YOU NEED BONDBUILDING HAIR CARE The Latest What Are Salt Bonds A salt molecule contains atoms of two elements: These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. Ionic bonding is the type of bonding that holds salts together. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. The. What Are Salt Bonds.
From www.researchgate.net
Salt bridge bonds between IMM01 and CD47. Download Scientific Diagram What Are Salt Bonds A salt molecule contains atoms of two elements: Ionic bonding is the type of bonding that holds salts together. In the case of table salt, the positively charged. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. The bonds in salt compounds are called ionic. What Are Salt Bonds.
From www.architecturalecologies.cca.edu
Salt Bonds — Architectural Ecologies Lab What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. These atoms arrange themselves into tidy cubes, with each sodium forming. What Are Salt Bonds.
From www.slideserve.com
PPT F 2006 BIOC 3405 PowerPoint Presentation, free download ID6037141 What Are Salt Bonds A salt molecule contains atoms of two elements: Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. These atoms arrange themselves into tidy. What Are Salt Bonds.
From www.pinterest.ca
The bonds are what keeps our hair together. We can temporarily destroy What Are Salt Bonds Ionic bonding is the type of bonding that holds salts together. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. A salt molecule. What Are Salt Bonds.
From stock.adobe.com
Structure of sodium chloride (salt).NaCl model.Vector illustration What Are Salt Bonds To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. A salt molecule contains atoms of two elements: These atoms arrange themselves into tidy. What Are Salt Bonds.
From www.researchgate.net
Salt bridge bonds between IMM01 and CD47. Download Scientific Diagram What Are Salt Bonds Ionic bonding is the type of bonding that holds salts together. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. In the case of table salt, the positively charged. To better understand why and how ions — atoms that have a charge. What Are Salt Bonds.
From opentextbc.ca
2.2 Bonding and Lattices Physical Geology What Are Salt Bonds In the case of table salt, the positively charged. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. A salt molecule contains atoms of two elements: Ionic bonding is the type of bonding that holds salts together. Salts are held together by. What Are Salt Bonds.
From www.slideserve.com
PPT The heme group. PowerPoint Presentation, free download ID1155734 What Are Salt Bonds Ionic bonding is the type of bonding that holds salts together. A salt molecule contains atoms of two elements: In the case of table salt, the positively charged. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. To better understand why and how ions — atoms that have a charge due to the. What Are Salt Bonds.
From www.slideserve.com
PPT Bonding to Olefins, Polyolefins and Alkynes PowerPoint What Are Salt Bonds To better understand why and how ions — atoms that have a charge due to the loss or gain of electrons — are formed,. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. In the case of table salt, the positively charged.. What Are Salt Bonds.
From www.slideserve.com
PPT Unit 8 Ionic Bonds PowerPoint Presentation, free download ID What Are Salt Bonds Ionic bonding is the type of bonding that holds salts together. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. In the case of table salt,. What Are Salt Bonds.
From socratic.org
What type of interaction is depicted by the dashed line? Socratic What Are Salt Bonds Ionic bonding is the type of bonding that holds salts together. These atoms arrange themselves into tidy cubes, with each sodium forming a chemical bond with a. In the case of table salt, the positively charged. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion. What Are Salt Bonds.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive What Are Salt Bonds Ionic bonding is the type of bonding that holds salts together. In the case of table salt, the positively charged. A salt molecule contains atoms of two elements: Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. To better understand why and how ions — atoms that. What Are Salt Bonds.
From owlcation.com
Primary and Secondary Bonds Owlcation What Are Salt Bonds The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. A salt molecule contains atoms of two elements: In the case of table salt, the positively charged. Salts are held together by ionic bonds, which are formed when a positively charged ion is. What Are Salt Bonds.
From www.slideserve.com
PPT By Kaila Amitoj Chopra, Jesse Johnson, Enrico Sagullo What Are Salt Bonds In the case of table salt, the positively charged. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. Ionic bonding. What Are Salt Bonds.
From www.slideserve.com
PPT PROPERTIES OF THE HAIR AND SCALP PowerPoint Presentation, free What Are Salt Bonds Ionic bonding is the type of bonding that holds salts together. Salts are held together by ionic bonds, which are formed when a positively charged ion is attracted to a negatively charged ion. The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged.. What Are Salt Bonds.