Chlorine Electron Configuration Class 11 . access detailed info on all elements: Use this to find the electronic configuration of chlorine. Chlorine belongs to group 17 which is known as. chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Atomic mass, electron configurations, charges, and more. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. View rotating bohr models for. Full ground state electron configuration: the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$.
from fyolklyyl.blob.core.windows.net
View rotating bohr models for. Full ground state electron configuration: Atomic mass, electron configurations, charges, and more. chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. Chlorine belongs to group 17 which is known as. access detailed info on all elements: Use this to find the electronic configuration of chlorine.
Chloride Ion Valence Electrons at David Lovato blog
Chlorine Electron Configuration Class 11 The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. access detailed info on all elements: Atomic mass, electron configurations, charges, and more. Use this to find the electronic configuration of chlorine. Full ground state electron configuration: The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. Chlorine belongs to group 17 which is known as. View rotating bohr models for.
From www.dreamstime.com
Electron of the Element Chlorine Stock Vector Illustration of Chlorine Electron Configuration Class 11 chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Chlorine belongs to group 17 which is known as. View rotating bohr models for. Use this to find the electronic configuration of chlorine. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. The noble gases. Chlorine Electron Configuration Class 11.
From www.youtube.com
Electron Configuration Diagram for Chlorine YouTube Chlorine Electron Configuration Class 11 Full ground state electron configuration: in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. access detailed info on all elements: chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Chlorine belongs to. Chlorine Electron Configuration Class 11.
From www.youtube.com
Cl(Chloride ion) and Cl(Chlorine) Electron Configuration,Orbital Chlorine Electron Configuration Class 11 chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Chlorine belongs to group 17 which is known as. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. View rotating bohr models for. in order to write. Chlorine Electron Configuration Class 11.
From orderdewaltdw718.blogspot.com
45 atomic orbital diagram for chlorine Diagram Online Chlorine Electron Configuration Class 11 Use this to find the electronic configuration of chlorine. Full ground state electron configuration: Atomic mass, electron configurations, charges, and more. Chlorine belongs to group 17 which is known as. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. in order to write the chlorine electron configuration we first need to know the number of electrons. Chlorine Electron Configuration Class 11.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Chlorine Electron Configuration Class 11 Full ground state electron configuration: Atomic mass, electron configurations, charges, and more. Use this to find the electronic configuration of chlorine. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and. Chlorine Electron Configuration Class 11.
From exyaxpzoa.blob.core.windows.net
Electron Configuration For Chlorine (Cl) at Deborah Morris blog Chlorine Electron Configuration Class 11 The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. chlorine has the electron configuration. Chlorine Electron Configuration Class 11.
From www.slideserve.com
PPT ELECTRONIC CONFIGURATION PowerPoint Presentation, free download Chlorine Electron Configuration Class 11 Chlorine belongs to group 17 which is known as. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. Full ground state electron configuration: chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. the electronic configuration of. Chlorine Electron Configuration Class 11.
From www.youtube.com
Electron Configuration of Chlorine Cl Lesson YouTube Chlorine Electron Configuration Class 11 the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. View rotating bohr models for. Use this to find the electronic configuration of chlorine. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. chlorine has the electron configuration [ne]3s23p5, with the seven electrons. Chlorine Electron Configuration Class 11.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Electron Configuration Class 11 Full ground state electron configuration: Chlorine belongs to group 17 which is known as. View rotating bohr models for. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. access detailed info on all elements: chlorine has the electron configuration [ne]3s23p5, with the seven electrons. Chlorine Electron Configuration Class 11.
From www.youtube.com
Chlorine Electronic Configuration YouTube Chlorine Electron Configuration Class 11 the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. Use this to find the electronic configuration of chlorine. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. View rotating bohr models for. in order to write the chlorine electron configuration we first need to know the. Chlorine Electron Configuration Class 11.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Electron Configuration Class 11 View rotating bohr models for. access detailed info on all elements: Chlorine belongs to group 17 which is known as. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. Use this to find the electronic configuration of chlorine. Atomic mass, electron configurations, charges, and more. Full ground state electron configuration: in order to write the. Chlorine Electron Configuration Class 11.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Electron Configuration Class 11 View rotating bohr models for. Full ground state electron configuration: the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. Atomic mass, electron configurations, charges, and more. Chlorine belongs to group 17 which is known as. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. Use this to. Chlorine Electron Configuration Class 11.
From byjus.com
Find out the number of valence electrons present in chlorine. Chlorine Electron Configuration Class 11 chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. View rotating bohr models for. Use this to find the electronic configuration of chlorine. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. the electronic configuration of. Chlorine Electron Configuration Class 11.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Electron Configuration Class 11 Chlorine belongs to group 17 which is known as. View rotating bohr models for. Use this to find the electronic configuration of chlorine. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. access detailed info on all elements: Full ground state electron configuration: Atomic mass,. Chlorine Electron Configuration Class 11.
From ar.inspiredpencil.com
Electron Configuration For Chlorine Chlorine Electron Configuration Class 11 View rotating bohr models for. Chlorine belongs to group 17 which is known as. Use this to find the electronic configuration of chlorine. access detailed info on all elements: Full ground state electron configuration: Atomic mass, electron configurations, charges, and more. in order to write the chlorine electron configuration we first need to know the number of electrons. Chlorine Electron Configuration Class 11.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Electron Configuration Class 11 View rotating bohr models for. Chlorine belongs to group 17 which is known as. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. Full ground state electron configuration: the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. in order to write the chlorine electron configuration we. Chlorine Electron Configuration Class 11.
From sciencenotes.org
Chlorine Facts Chlorine Electron Configuration Class 11 Use this to find the electronic configuration of chlorine. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. access detailed info on all elements: Chlorine belongs to group 17 which is known as. The noble gases (with the exception of helium which has a duplet. Chlorine Electron Configuration Class 11.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? Chlorine Electron Configuration Class 11 access detailed info on all elements: The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. View rotating bohr models for. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Chlorine belongs to group 17 which. Chlorine Electron Configuration Class 11.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Electron Configuration Class 11 Chlorine belongs to group 17 which is known as. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. View rotating bohr models for. Use this to find the electronic configuration of chlorine. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. Atomic mass, electron configurations, charges, and. Chlorine Electron Configuration Class 11.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of Chlorine Electron Configuration Class 11 Full ground state electron configuration: access detailed info on all elements: Atomic mass, electron configurations, charges, and more. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. View rotating bohr models for. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. chlorine has the electron. Chlorine Electron Configuration Class 11.
From egpat.com
Lewis dot structure How to write? Chlorine Electron Configuration Class 11 Use this to find the electronic configuration of chlorine. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. Atomic mass, electron configurations, charges, and more. Chlorine belongs. Chlorine Electron Configuration Class 11.
From animalia-life.club
Chlorine Electron Configuration Chlorine Electron Configuration Class 11 Full ground state electron configuration: The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Atomic mass, electron configurations, charges, and more. the electronic configuration of sodium is. Chlorine Electron Configuration Class 11.
From fyolklyyl.blob.core.windows.net
Chloride Ion Valence Electrons at David Lovato blog Chlorine Electron Configuration Class 11 View rotating bohr models for. in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Full ground state electron configuration: Use this to find the. Chlorine Electron Configuration Class 11.
From www.youtube.com
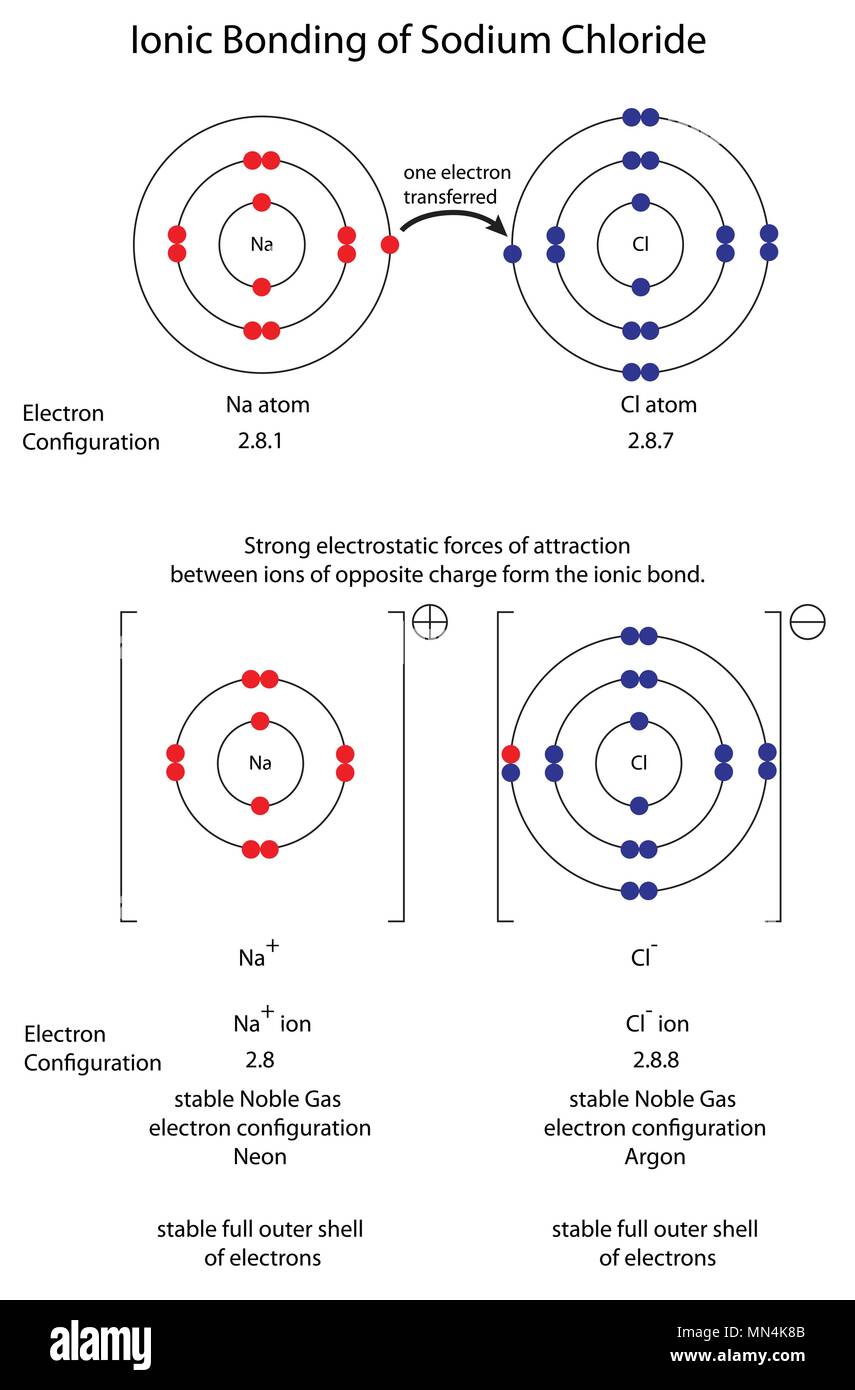
(a) Give the electronic configurations of sodium and chlorine. (b Chlorine Electron Configuration Class 11 The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. access detailed info on all elements: in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Full ground state electron configuration: Chlorine belongs to group 17 which. Chlorine Electron Configuration Class 11.
From www.youtube.com
Chlorine electronic configuration How to Write Chlorine electronic Chlorine Electron Configuration Class 11 Use this to find the electronic configuration of chlorine. access detailed info on all elements: The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Full ground state. Chlorine Electron Configuration Class 11.
From www.schoolmykids.com
Cl Chlorine Element Information Facts, Properties, Trends, Uses and Chlorine Electron Configuration Class 11 Full ground state electron configuration: Use this to find the electronic configuration of chlorine. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. Atomic mass, electron configurations, charges, and more. Chlorine belongs to group 17 which is known as. in order to write the chlorine electron configuration we first. Chlorine Electron Configuration Class 11.
From byjus.com
Electronic Configuration Distribution of Electrons Chemistry Chlorine Electron Configuration Class 11 in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the. Chlorine Electron Configuration Class 11.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electron Configuration Class 11 Full ground state electron configuration: in order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there. Use this to find the electronic configuration of chlorine. Chlorine belongs to group 17 which is known as. The noble gases (with the exception of helium which has a duplet of electrons). Chlorine Electron Configuration Class 11.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Electron Configuration Class 11 chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. access detailed info on all elements: Chlorine belongs to group 17 which is known as. Use this to find the electronic configuration of chlorine. The. Chlorine Electron Configuration Class 11.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Electron Configuration Class 11 chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Chlorine belongs to group 17 which is known as. View rotating bohr models for. access detailed info on all elements: Atomic mass, electron configurations, charges, and more. Use this to find the electronic configuration of chlorine. . Chlorine Electron Configuration Class 11.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Electron Configuration Class 11 Use this to find the electronic configuration of chlorine. Full ground state electron configuration: access detailed info on all elements: Chlorine belongs to group 17 which is known as. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. in order to write the chlorine electron configuration we first need to know the number of electrons. Chlorine Electron Configuration Class 11.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Electron Configuration Class 11 Chlorine belongs to group 17 which is known as. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. View rotating bohr models for. Use this to find the electronic configuration of chlorine. access detailed info on all elements: Full ground state electron configuration: the electronic configuration of sodium. Chlorine Electron Configuration Class 11.
From www.youtube.com
Chlorine Ground State Electron Configuration YouTube Chlorine Electron Configuration Class 11 Use this to find the electronic configuration of chlorine. View rotating bohr models for. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. the electronic configuration of. Chlorine Electron Configuration Class 11.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Electron Configuration Class 11 the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. access detailed info on all elements: chlorine has the electron configuration [ne]3s23p5, with the seven electrons serving as its valence electrons in the third and outermost shell. Use this to find the electronic configuration of chlorine. Full ground state electron configuration: Atomic mass, electron configurations, charges,. Chlorine Electron Configuration Class 11.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Electron Configuration Class 11 Chlorine belongs to group 17 which is known as. the electronic configuration of sodium is $1 {s^2}2 {s^2}2 {p^6}3 {s^1}$. The noble gases (with the exception of helium which has a duplet of electrons) have a particularly stable outer. Use this to find the electronic configuration of chlorine. access detailed info on all elements: Full ground state electron. Chlorine Electron Configuration Class 11.