Quality Assurance In Sterilization . In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. Proper sterilization of instruments and materials is a critical aspect of infection control. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). Other specifications may include sterilant residues and. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle.
from capstone.isye.gatech.edu
Other specifications may include sterilant residues and. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. Proper sterilization of instruments and materials is a critical aspect of infection control. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for.
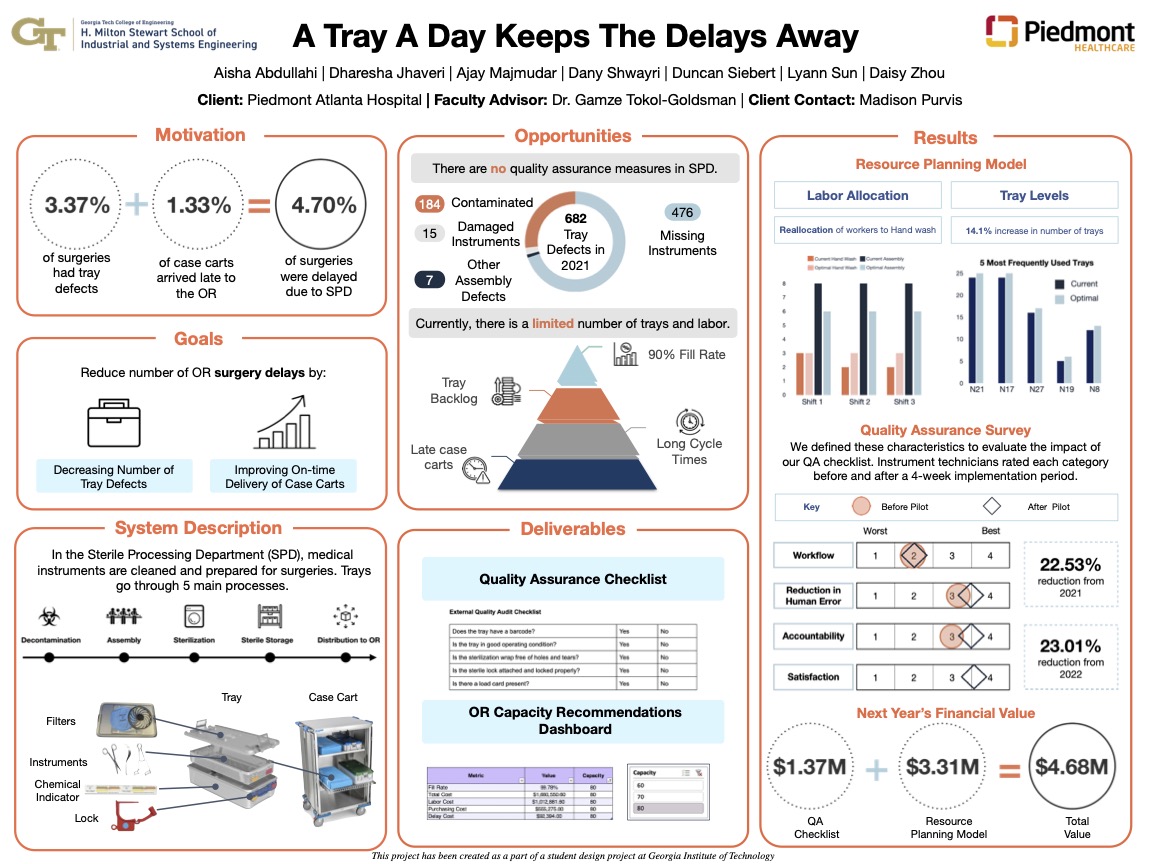
Sterile Processing Department Quality Assurance and Resource Allocation
Quality Assurance In Sterilization Other specifications may include sterilant residues and. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). Other specifications may include sterilant residues and. In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Proper sterilization of instruments and materials is a critical aspect of infection control.
From www.labcon.com
Labcon Sterility Quality Assurance In Sterilization Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. For sterilization processes,. Quality Assurance In Sterilization.
From www.scribd.com
Lyophilized BMR PDF Quality Assurance Sterilization (Microbiology) Quality Assurance In Sterilization Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Proper sterilization of instruments and materials is a critical aspect of infection control. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Biological and chemical indicator testing is also done. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Monitoring the Sterilization Process PowerPoint Presentation Quality Assurance In Sterilization Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. Sterility. Quality Assurance In Sterilization.
From www.sterilization-baltics.com
Quality Assurance Quality Assurance In Sterilization Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). This article series outlines the key. Quality Assurance In Sterilization.
From pharmaguidances.com
STERILITY ASSURANCE Quality Assurance In Sterilization Other specifications may include sterilant residues and. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide. Quality Assurance In Sterilization.
From wecarepharm.com
Quality Assurance WeCare Pharmacy Quality Assurance In Sterilization Other specifications may include sterilant residues and. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. This article series outlines the key components. Quality Assurance In Sterilization.
From cssdtechnicianhub.com
sterileprocessingqualityassurance CSSD Technician Hub Quality Assurance In Sterilization To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. In many cases, quality/sterility. Quality Assurance In Sterilization.
From cssdtechnicianhub.com
Central Sterile Processing and Distribution CSSD Technician Hub Quality Assurance In Sterilization Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Cleaning, Packaging and Sterilization of Instruments PowerPoint Quality Assurance In Sterilization Other specifications may include sterilant residues and. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Proper sterilization of instruments and materials is a critical aspect of infection control. Quality. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Monitoring the Sterilization Process PowerPoint Presentation Quality Assurance In Sterilization For sterilization processes, the primary device specification is the desired sterility assurance level (sal). To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle.. Quality Assurance In Sterilization.
From autoclave.se
Autoclave ISYESON 18 Liter Aison International Quality Assurance In Sterilization Other specifications may include sterilant residues and. In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Quality assurance in sterile processing is. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Approvisionement Nord Ouest PowerPoint Presentation, free Quality Assurance In Sterilization For sterilization processes, the primary device specification is the desired sterility assurance level (sal). In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Cleaning, Packaging and Sterilization of Instruments PowerPoint Quality Assurance In Sterilization Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Other specifications may include sterilant residues and. Proper sterilization of instruments and materials is a critical aspect of infection control. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. In many cases, quality/sterility assurance. Quality Assurance In Sterilization.
From pharmacyconnection.ca
Focus on Quality Assurance Tips on Meeting Sterile Compounding Quality Assurance In Sterilization This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Other specifications may include sterilant residues and. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the. Quality Assurance In Sterilization.
From dokumen.tips
(PDF) QUALITY ASSURANCE IN STERILIZATION · routine control of a Quality Assurance In Sterilization This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Other specifications may include sterilant residues and. In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Cleaning/decontamination, disinfection, and sterilization Quality Assurance In Sterilization To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Proper sterilization of instruments and materials is a critical aspect of infection control. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Other. Quality Assurance In Sterilization.
From kerone.com
Different Types of Sterilization Process Quality Assurance In Sterilization Proper sterilization of instruments and materials is a critical aspect of infection control. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. This. Quality Assurance In Sterilization.
From mms.mckesson.com
Sterilization packaging & quality assurance McKesson MedicalSurgical Quality Assurance In Sterilization Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Proper sterilization of instruments and materials is a critical aspect of infection control. In many cases, quality/sterility assurance best practices. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Sterilization and validation PowerPoint Presentation, free Quality Assurance In Sterilization Other specifications may include sterilant residues and. Proper sterilization of instruments and materials is a critical aspect of infection control. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). Biological and chemical indicator testing is also done for ongoing. Quality Assurance In Sterilization.
From hrmedical.en.made-in-china.com
Quality Assurance Easy Peel Self Sealing Sterilization Pouches Quality Assurance In Sterilization This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. To ensure. Quality Assurance In Sterilization.
From www.labcon.com
Labcon Sterility Quality Assurance In Sterilization Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. In many cases, quality/sterility assurance best practices can boost process efficiency as. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Cleaning/decontamination, disinfection, and sterilization Quality Assurance In Sterilization This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide. Quality Assurance In Sterilization.
From www.burkhartdental.com
Sterilization Monitoring An Important Quality Assurance Process Quality Assurance In Sterilization Proper sterilization of instruments and materials is a critical aspect of infection control. Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to.. Quality Assurance In Sterilization.
From www.researchgate.net
(PDF) Quality Assurance for Sterile Products Quality Assurance In Sterilization In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. Other specifications may include sterilant residues and. To ensure the best possible patient outcomes, and successful surveys, it. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Disinfection and Sterilization PowerPoint Presentation, free Quality Assurance In Sterilization This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Proper sterilization of instruments and materials is a critical aspect of infection control. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. Quality assurance in sterile processing is a meticulous blend of established protocols, continuous. Quality Assurance In Sterilization.
From capstone.isye.gatech.edu
Sterile Processing Department Quality Assurance and Resource Allocation Quality Assurance In Sterilization In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). Proper sterilization of instruments and materials is a critical aspect of infection control. To ensure the best possible patient outcomes, and successful. Quality Assurance In Sterilization.
From www.slideserve.com
PPT Principles of Disinfection and Sterilization in the dental Quality Assurance In Sterilization Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Proper sterilization of instruments and materials is a critical aspect of infection control. Sterility assurance products including biological indicators (bi). Quality Assurance In Sterilization.
From www.scribd.com
1 Quality Assurance Manual for Sterilisation Services Audit Quality Quality Assurance In Sterilization Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. For sterilization processes,. Quality Assurance In Sterilization.
From theaahp.org
Safe Drugs, Happy Consumers Sterility Assurance Programs The Quality Assurance In Sterilization Proper sterilization of instruments and materials is a critical aspect of infection control. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide you the confidence that the sterilizer is functioning properly and cycle. Other specifications may include sterilant residues and. Quality assurance in. Quality Assurance In Sterilization.
From present5.com
Monitoring the Sterilization Process THETA CHAPTER May 09 Quality Assurance In Sterilization For sterilization processes, the primary device specification is the desired sterility assurance level (sal). Other specifications may include sterilant residues and. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. Sterility assurance products including biological. Quality Assurance In Sterilization.
From www.slideserve.com
PPT MEDICATION SAFETY PowerPoint Presentation, free download ID4568029 Quality Assurance In Sterilization In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Other specifications may include sterilant residues and. For sterilization processes, the primary device. Quality Assurance In Sterilization.
From app.docseducation.com
Sterilization Monitoring; An Important Quality Assurance Process DOCS Quality Assurance In Sterilization Proper sterilization of instruments and materials is a critical aspect of infection control. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. Sterility assurance products including biological indicators. Quality Assurance In Sterilization.
From www.scribd.com
9020 Quality AssuranceQuality Control (1997) PDF Quality Assurance Quality Assurance In Sterilization Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. This article series outlines the key components of sterility assurance, including the validation of sterilization processes,. For sterilization processes, the primary device specification is the desired sterility assurance level (sal). Sterility assurance products including biological indicators (bi) and chemical indicators (ci) provide. Quality Assurance In Sterilization.
From www.burkhartdental.com
Sterilization Monitoring An Important Quality Assurance Process Quality Assurance In Sterilization Quality assurance in sterile processing is a meticulous blend of established protocols, continuous monitoring, and an unwavering. Proper sterilization of instruments and materials is a critical aspect of infection control. Biological and chemical indicator testing is also done for ongoing quality assurance testing of representative samples of actual. This article series outlines the key components of sterility assurance, including the. Quality Assurance In Sterilization.
From www.scribd.com
Quality Assurance in Microbiology Labs PDF Quality Assurance Quality Assurance In Sterilization In many cases, quality/sterility assurance best practices can boost process efficiency as well, enabling the cs/spd to deliver safer and more effective instruments to. To ensure the best possible patient outcomes, and successful surveys, it is important to have a robust quality assurance program in place for. For sterilization processes, the primary device specification is the desired sterility assurance level. Quality Assurance In Sterilization.