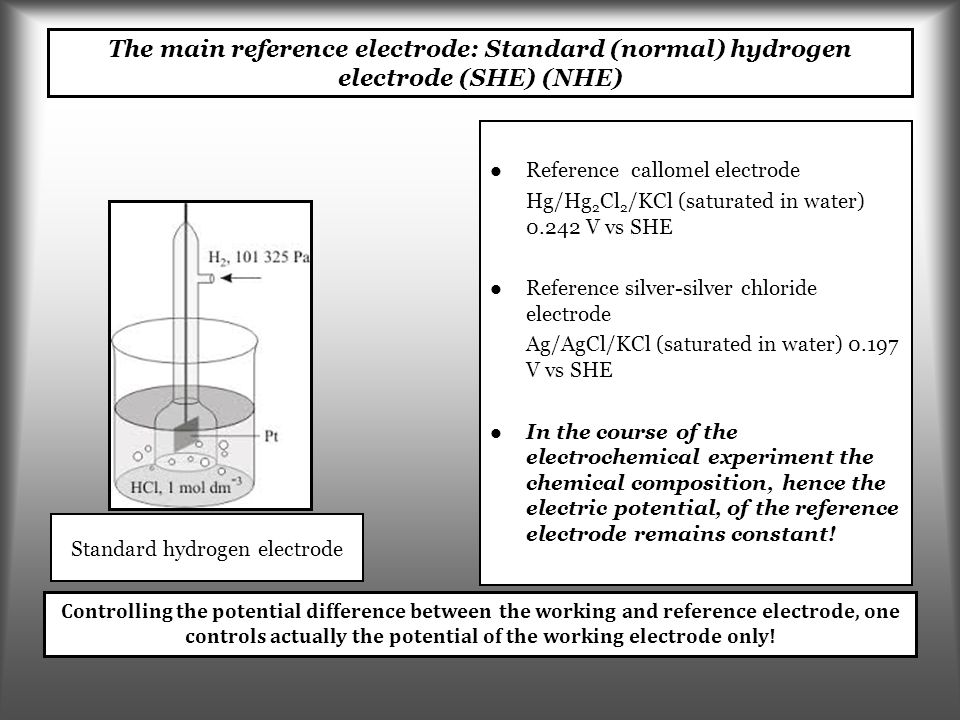
Hydrogen Electrode Ppt . An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. What is a standard hydrogen electrode? Which statement is correct regarding the potential energy of an electron at this electrode? An electrode has a negative electrode potential. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Lithium ions in aqueous solution have the weakest tendency to gain. The hydrogen electrode is used as a reference for electrode potential measurements. Those with positive values gain electrons more easily than hydrogen ions.
from slideplayer.com
An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. The hydrogen electrode is used as a reference for electrode potential measurements. Which statement is correct regarding the potential energy of an electron at this electrode? Those with positive values gain electrons more easily than hydrogen ions. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. What is a standard hydrogen electrode? Lithium ions in aqueous solution have the weakest tendency to gain. An electrode has a negative electrode potential.
Introduction to electrochemical techniques ppt download
Hydrogen Electrode Ppt The hydrogen electrode is used as a reference for electrode potential measurements. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The hydrogen electrode is used as a reference for electrode potential measurements. Lithium ions in aqueous solution have the weakest tendency to gain. Those with positive values gain electrons more easily than hydrogen ions. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Which statement is correct regarding the potential energy of an electron at this electrode? What is a standard hydrogen electrode? An electrode has a negative electrode potential.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Hydrogen Electrode Ppt An electrode has a negative electrode potential. Which statement is correct regarding the potential energy of an electron at this electrode? An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential. Hydrogen Electrode Ppt.
From byjus.com
What is standard hydrogen electrode? Hydrogen Electrode Ppt Which statement is correct regarding the potential energy of an electron at this electrode? Lithium ions in aqueous solution have the weakest tendency to gain. What is a standard hydrogen electrode? The hydrogen electrode is used as a reference for electrode potential measurements. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Hydrogen Electrode Ppt An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Which statement is correct regarding the potential energy of an electron at this electrode? An electrode has a negative electrode potential. What is a standard hydrogen electrode? Those with positive values gain electrons more easily than. Hydrogen Electrode Ppt.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use Hydrogen Electrode Ppt What is a standard hydrogen electrode? An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. An electrode has a negative electrode potential. The hydrogen electrode is used as a reference for electrode potential measurements. Which statement is correct regarding the potential energy of an electron. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5744606 Hydrogen Electrode Ppt An electrode has a negative electrode potential. Those with positive values gain electrons more easily than hydrogen ions. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. What is a standard hydrogen electrode? Standard hydrogen electrode is used as a reference electrode when calculating the. Hydrogen Electrode Ppt.
From saylordotorg.github.io
Standard Potentials Hydrogen Electrode Ppt An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Which statement is correct regarding the potential energy of an electron at this electrode? The hydrogen electrode is used as a reference for electrode potential measurements. Lithium ions in aqueous solution have the weakest tendency to. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Hydrogen Electrode Ppt Lithium ions in aqueous solution have the weakest tendency to gain. What is a standard hydrogen electrode? Which statement is correct regarding the potential energy of an electron at this electrode? An electrode has a negative electrode potential. The hydrogen electrode is used as a reference for electrode potential measurements. Those with positive values gain electrons more easily than hydrogen. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free Hydrogen Electrode Ppt Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. What is a standard hydrogen electrode? The hydrogen electrode is used as a reference for electrode potential measurements. Those with positive values gain electrons more easily than hydrogen ions. An ideal reference electrode has a reproducible and stable potential that is. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT CHEM 160 General Chemistry II Lecture Presentation Hydrogen Electrode Ppt The hydrogen electrode is used as a reference for electrode potential measurements. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Lithium ions in aqueous solution have the weakest tendency to gain. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID4435141 Hydrogen Electrode Ppt Which statement is correct regarding the potential energy of an electron at this electrode? Those with positive values gain electrons more easily than hydrogen ions. Lithium ions in aqueous solution have the weakest tendency to gain. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition.. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Hydrogen Electrode Ppt Those with positive values gain electrons more easily than hydrogen ions. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Which statement is correct regarding the potential energy of an electron at this electrode? An ideal reference electrode has a reproducible and stable potential that is not affected by small. Hydrogen Electrode Ppt.
From mungfali.com
Ppt Chapter 17 Electrochemistry Powerpoint Presentation, Free A12 Hydrogen Electrode Ppt What is a standard hydrogen electrode? The hydrogen electrode is used as a reference for electrode potential measurements. Those with positive values gain electrons more easily than hydrogen ions. Lithium ions in aqueous solution have the weakest tendency to gain. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. An. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Electrochemical Theory PowerPoint Presentation, free download Hydrogen Electrode Ppt Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Which statement is correct regarding the potential energy of an electron at this electrode? Those with positive values gain electrons more easily than hydrogen ions. An ideal reference electrode has a reproducible and stable potential that is not affected by small. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT BASIC ELECTROCHEMISTRY PowerPoint Presentation ID594481 Hydrogen Electrode Ppt Those with positive values gain electrons more easily than hydrogen ions. Lithium ions in aqueous solution have the weakest tendency to gain. What is a standard hydrogen electrode? The hydrogen electrode is used as a reference for electrode potential measurements. An electrode has a negative electrode potential. Which statement is correct regarding the potential energy of an electron at this. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Hydrogen Electrode Ppt What is a standard hydrogen electrode? Those with positive values gain electrons more easily than hydrogen ions. The hydrogen electrode is used as a reference for electrode potential measurements. An electrode has a negative electrode potential. Lithium ions in aqueous solution have the weakest tendency to gain. Which statement is correct regarding the potential energy of an electron at this. Hydrogen Electrode Ppt.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Hydrogen Electrode Ppt The hydrogen electrode is used as a reference for electrode potential measurements. What is a standard hydrogen electrode? An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Those with positive values gain electrons more easily than hydrogen ions. Lithium ions in aqueous solution have the. Hydrogen Electrode Ppt.
From slideplayer.com
Introduction to electrochemical techniques ppt download Hydrogen Electrode Ppt Lithium ions in aqueous solution have the weakest tendency to gain. An electrode has a negative electrode potential. What is a standard hydrogen electrode? Which statement is correct regarding the potential energy of an electron at this electrode? The hydrogen electrode is used as a reference for electrode potential measurements. Those with positive values gain electrons more easily than hydrogen. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID6733376 Hydrogen Electrode Ppt Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Those with positive values gain electrons more easily than hydrogen ions. Which statement is correct regarding the potential energy of an electron at this electrode? The hydrogen electrode is used as a reference for electrode potential measurements. What is a standard. Hydrogen Electrode Ppt.
From question.pandai.org
Standard Electrode Potential Hydrogen Electrode Ppt Which statement is correct regarding the potential energy of an electron at this electrode? The hydrogen electrode is used as a reference for electrode potential measurements. What is a standard hydrogen electrode? Those with positive values gain electrons more easily than hydrogen ions. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a. Hydrogen Electrode Ppt.
From dokumen.tips
(PPT) STANDARD ELECTRODE POTENTIALS. THE STANDARD HYDROGEN ELECTRODE In Hydrogen Electrode Ppt Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. The hydrogen electrode is used as a reference for electrode potential measurements. Lithium ions in aqueous solution. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Hydrogen Electrode Ppt Those with positive values gain electrons more easily than hydrogen ions. Which statement is correct regarding the potential energy of an electron at this electrode? An electrode has a negative electrode potential. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. What is a standard. Hydrogen Electrode Ppt.
From stock.adobe.com
Standard hydrogen electrode diagram. Scientific vector illustration Hydrogen Electrode Ppt An electrode has a negative electrode potential. What is a standard hydrogen electrode? Those with positive values gain electrons more easily than hydrogen ions. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Lithium ions in aqueous solution have the weakest tendency to gain. Standard. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Hydrogen Electrode Ppt An electrode has a negative electrode potential. The hydrogen electrode is used as a reference for electrode potential measurements. What is a standard hydrogen electrode? Which statement is correct regarding the potential energy of an electron at this electrode? Those with positive values gain electrons more easily than hydrogen ions. Lithium ions in aqueous solution have the weakest tendency to. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Hydrogen Electrode Ppt An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Those with positive values gain electrons more easily than hydrogen ions. An electrode has a negative electrode. Hydrogen Electrode Ppt.
From monomole.com
Standard hydrogen electrode Mono Mole Hydrogen Electrode Ppt The hydrogen electrode is used as a reference for electrode potential measurements. What is a standard hydrogen electrode? An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. Those with positive values gain electrons more easily than hydrogen ions. Which statement is correct regarding the potential. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2012118 Hydrogen Electrode Ppt Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The hydrogen electrode is used as a reference for electrode potential measurements. An electrode has a negative electrode potential. Which statement is correct regarding the potential energy of an electron at this electrode? An ideal reference electrode has a reproducible and. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Hydrogen Electrode Ppt What is a standard hydrogen electrode? The hydrogen electrode is used as a reference for electrode potential measurements. An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in temperature or solution composition. An electrode has a negative electrode potential. Lithium ions in aqueous solution have the weakest tendency to gain.. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT REDOX EQM Standard Hydrogen Electrode and Measurement of Hydrogen Electrode Ppt What is a standard hydrogen electrode? An electrode has a negative electrode potential. Lithium ions in aqueous solution have the weakest tendency to gain. Which statement is correct regarding the potential energy of an electron at this electrode? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Those with positive. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT STANDARD REDUCTION POTENTIAL PowerPoint Presentation, free Hydrogen Electrode Ppt Those with positive values gain electrons more easily than hydrogen ions. Which statement is correct regarding the potential energy of an electron at this electrode? Lithium ions in aqueous solution have the weakest tendency to gain. An electrode has a negative electrode potential. What is a standard hydrogen electrode? Standard hydrogen electrode is used as a reference electrode when calculating. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Ch. 18 Electrochemistry PowerPoint Presentation, free download Hydrogen Electrode Ppt An electrode has a negative electrode potential. Which statement is correct regarding the potential energy of an electron at this electrode? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. What is a standard hydrogen electrode? An ideal reference electrode has a reproducible and stable potential that is not affected. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Hydrogen Electrode Ppt An electrode has a negative electrode potential. Those with positive values gain electrons more easily than hydrogen ions. The hydrogen electrode is used as a reference for electrode potential measurements. Which statement is correct regarding the potential energy of an electron at this electrode? Lithium ions in aqueous solution have the weakest tendency to gain. Standard hydrogen electrode is used. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Chemistry 100 Chapter 20 PowerPoint Presentation, free download Hydrogen Electrode Ppt Those with positive values gain electrons more easily than hydrogen ions. Lithium ions in aqueous solution have the weakest tendency to gain. The hydrogen electrode is used as a reference for electrode potential measurements. What is a standard hydrogen electrode? An ideal reference electrode has a reproducible and stable potential that is not affected by small currents or changes in. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID6102398 Hydrogen Electrode Ppt Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. What is a standard hydrogen electrode? Which statement is correct regarding the potential energy of an electron at this electrode? Lithium ions in aqueous solution have the weakest tendency to gain. Those with positive values gain electrons more easily than hydrogen. Hydrogen Electrode Ppt.
From en.ppt-online.org
Electrochemistry. Oxidationreduction equilibrium in water solutions Hydrogen Electrode Ppt Those with positive values gain electrons more easily than hydrogen ions. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. An electrode has a negative electrode potential. What is a standard hydrogen electrode? Lithium ions in aqueous solution have the weakest tendency to gain. The hydrogen electrode is used as. Hydrogen Electrode Ppt.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID945046 Hydrogen Electrode Ppt Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Lithium ions in aqueous solution have the weakest tendency to gain. What is a standard hydrogen electrode? An electrode has a negative electrode potential. Those with positive values gain electrons more easily than hydrogen ions. The hydrogen electrode is used as. Hydrogen Electrode Ppt.