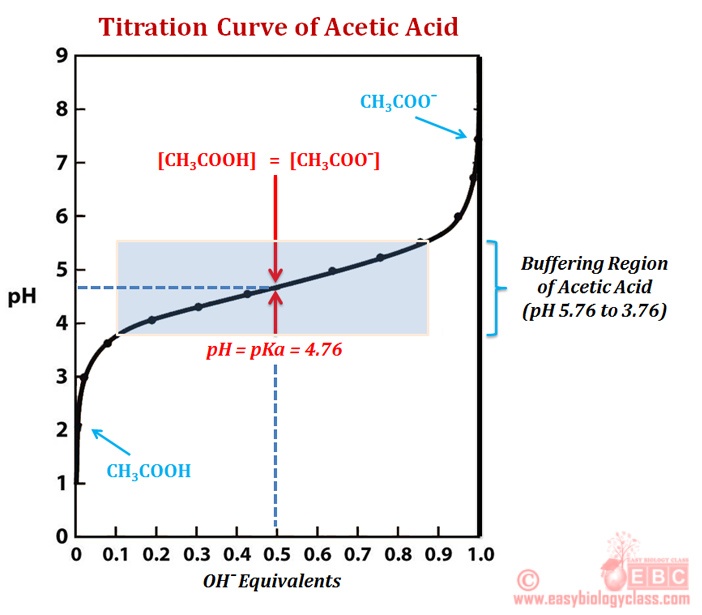
Tris Buffer Titration Curve . Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. The titration is monitored by potentiometric. With a moderate amount of naoh added, we have a solution that contains. Example curve of a titer determination of hydrochloric acid with tris as primary standard. The concentrations of tris and tes buffer were 1. Tris buffer, a weak base, dissociates in water to form tris ions and. Choosing the right biological buffer. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Influence of tris buffer on ph and titration curve.
from mungfali.com
The titration is monitored by potentiometric. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Choosing the right biological buffer. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Tris buffer, a weak base, dissociates in water to form tris ions and. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. With a moderate amount of naoh added, we have a solution that contains. The concentrations of tris and tes buffer were 1. Integrated heat plots for titration of tris (a) and tes (b) with metal ions.
Buffer Region On Titration Curve
Tris Buffer Titration Curve Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: With a moderate amount of naoh added, we have a solution that contains. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Choosing the right biological buffer. Influence of tris buffer on ph and titration curve. The concentrations of tris and tes buffer were 1. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Tris buffer, a weak base, dissociates in water to form tris ions and. Integrated heat plots for titration of tris (a) and tes (b) with metal ions. The titration is monitored by potentiometric.
From www.researchgate.net
Emission titration of 1 with CT DNA in Tris buffer [1] =5 mM, [DNA Tris Buffer Titration Curve Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: The titration is monitored by potentiometric. With a moderate amount of naoh added, we have a solution that contains. Choosing the right biological buffer. The concentrations of tris and tes buffer were 1. Integrated heat plots for titration of tris. Tris Buffer Titration Curve.
From mungfali.com
Buffer Region On Titration Curve Tris Buffer Titration Curve Integrated heat plots for titration of tris (a) and tes (b) with metal ions. The titration is monitored by potentiometric. Influence of tris buffer on ph and titration curve. With a moderate amount of naoh added, we have a solution that contains. The concentrations of tris and tes buffer were 1. Titration curve for the titration of 20 ml of. Tris Buffer Titration Curve.
From mavink.com
Titration Labeled Tris Buffer Titration Curve Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Choosing the right biological buffer. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Buffer titration. Tris Buffer Titration Curve.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Tris Buffer Titration Curve Choosing the right biological buffer. With a moderate amount of naoh added, we have a solution that contains. Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Influence of tris buffer on ph and titration curve. The titration is monitored by potentiometric. Buffer titration the following table can be used to determine the appropriate mix. Tris Buffer Titration Curve.
From www.researchgate.net
(a) Fluorescence titration (λexc = 360 nm) of L (2 mL, Tris buffer Tris Buffer Titration Curve The concentrations of tris and tes buffer were 1. The titration is monitored by potentiometric. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Example curve of a titer determination of hydrochloric acid with tris as primary standard. With a moderate amount. Tris Buffer Titration Curve.
From mavink.com
Buffer Region Titration Curve Tris Buffer Titration Curve Integrated heat plots for titration of tris (a) and tes (b) with metal ions. The concentrations of tris and tes buffer were 1. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. With a moderate amount of naoh added, we have a. Tris Buffer Titration Curve.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Tris Buffer Titration Curve The concentrations of tris and tes buffer were 1. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Example curve of a titer determination of hydrochloric acid with tris as primary standard. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris. Tris Buffer Titration Curve.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Tris Buffer Titration Curve Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Influence of tris buffer on ph and titration curve. Example curve of a titer determination of hydrochloric acid with. Tris Buffer Titration Curve.
From www.researchgate.net
Titration spectra of Cu2La complex (100 μM) in 50/50 V/V DMSO/Tris Tris Buffer Titration Curve The titration is monitored by potentiometric. With a moderate amount of naoh added, we have a solution that contains. Choosing the right biological buffer. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Choose a buffer based on your ph requirements as. Tris Buffer Titration Curve.
From ar.inspiredpencil.com
Titration Curve Buffer Region Tris Buffer Titration Curve Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Tris buffer, a weak base, dissociates in water to form tris ions and. Buffer titration the following table can be used to determine the appropriate mix of. Tris Buffer Titration Curve.
From www.researchgate.net
Absorption spectral changes of Pd1 [20 µM] in Tris buffer solution at Tris Buffer Titration Curve The titration is monitored by potentiometric. Example curve of a titer determination of hydrochloric acid with tris as primary standard. The concentrations of tris and tes buffer were 1. Influence of tris buffer on ph and titration curve. Choosing the right biological buffer. With a moderate amount of naoh added, we have a solution that contains. Integrated heat plots for. Tris Buffer Titration Curve.
From mungfali.com
Titration Curve Graph Tris Buffer Titration Curve The concentrations of tris and tes buffer were 1. The titration is monitored by potentiometric. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Tris buffer, a weak base, dissociates in water to form tris ions and. Example curve of a titer. Tris Buffer Titration Curve.
From www.researchgate.net
Standard curve for the acetone system Tris buffer. Download Tris Buffer Titration Curve With a moderate amount of naoh added, we have a solution that contains. Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Titration curve for the titration of. Tris Buffer Titration Curve.
From ar.inspiredpencil.com
Titration Curve Labeled Tris Buffer Titration Curve Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Choosing the right biological buffer. Influence of tris buffer on ph and titration curve. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that.. Tris Buffer Titration Curve.
From chart-studio.plotly.com
Titration of Phosphate Buffer with Base scatter chart made by Tris Buffer Titration Curve Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. The titration is monitored by potentiometric. Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric. Tris Buffer Titration Curve.
From www.researchgate.net
Potentiometric pH titration curve for (A) E4.TRIS ( 10 ), (B) D4.TRIS Tris Buffer Titration Curve With a moderate amount of naoh added, we have a solution that contains. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Influence of tris buffer on ph and titration curve. The concentrations of tris and tes buffer were 1. Choose a. Tris Buffer Titration Curve.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner Tris Buffer Titration Curve Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Choosing the right biological buffer. With a moderate amount of naoh added, we have a solution that contains. Integrated heat plots for titration of tris (a) and tes (b) with metal ions. The. Tris Buffer Titration Curve.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Tris Buffer Titration Curve Tris buffer, a weak base, dissociates in water to form tris ions and. The concentrations of tris and tes buffer were 1. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Example curve of a. Tris Buffer Titration Curve.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Tris Buffer Titration Curve Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: With a moderate amount of naoh added, we have a solution that contains. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Buffer titration the following table can be used to determine the appropriate mix. Tris Buffer Titration Curve.
From www.researchgate.net
CD titration of 22AG with complex in 10 mM Tris buffer. CD titration Tris Buffer Titration Curve Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Titration curve for the titration of 20 ml of 0.88 m acetic acid. Tris Buffer Titration Curve.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Tris Buffer Titration Curve Influence of tris buffer on ph and titration curve. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Choosing the right biological buffer. Integrated heat plots for titration of tris (a) and tes (b) with metal ions. The titration is monitored by potentiometric. Choose a buffer based on your ph requirements as well as the. Tris Buffer Titration Curve.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Tris Buffer Titration Curve Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: The titration is monitored by potentiometric. Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m. Tris Buffer Titration Curve.
From users.highland.edu
Buffers Tris Buffer Titration Curve Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Choosing the right biological buffer. The concentrations of tris and tes buffer were 1. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Tris buffer, a weak base, dissociates in water to form tris ions and.. Tris Buffer Titration Curve.
From forums.studentdoctor.net
Confusion about titration of polyprotic acids Student Doctor Network Tris Buffer Titration Curve Tris buffer, a weak base, dissociates in water to form tris ions and. The titration is monitored by potentiometric. Influence of tris buffer on ph and titration curve. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Integrated heat plots for titration. Tris Buffer Titration Curve.
From ar.inspiredpencil.com
Titration Curve Buffer Region Tris Buffer Titration Curve Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Example curve of a titer determination of hydrochloric acid with tris as primary. Tris Buffer Titration Curve.
From www.youtube.com
Buffers and Titration Curves YouTube Tris Buffer Titration Curve Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. The concentrations of tris and tes buffer were 1. The titration is monitored by potentiometric. Integrated heat plots for titration of tris (a) and tes (b) with metal ions. With a moderate amount. Tris Buffer Titration Curve.
From pilgaard.info
Acids and bases Buffers Michael Pilgaard's Chemistry Tris Buffer Titration Curve Influence of tris buffer on ph and titration curve. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Example curve of a titer determination of hydrochloric acid with tris as primary standard. Choose a buffer based on your ph requirements as well as the pka, a measure of acid. Tris Buffer Titration Curve.
From www.researchgate.net
pH titration curves of TRIS family (a) and morpholine family (b) at [T Tris Buffer Titration Curve With a moderate amount of naoh added, we have a solution that contains. The concentrations of tris and tes buffer were 1. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Choosing the right biological buffer. Choose a buffer based on your ph requirements as well as the pka,. Tris Buffer Titration Curve.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Tris Buffer Titration Curve The concentrations of tris and tes buffer were 1. Integrated heat plots for titration of tris (a) and tes (b) with metal ions. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Choosing the right biological buffer. Tris buffer, a weak base, dissociates in water to form tris ions. Tris Buffer Titration Curve.
From www.researchgate.net
pH titration curves of Cr(III) with TRIS family (a) and morpholine Tris Buffer Titration Curve Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. With a moderate amount of naoh added, we have a solution that contains. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Buffer titration the following table can be used to determine the appropriate mix of. Tris Buffer Titration Curve.
From socratic.org
Titration curve? Please help... Socratic Tris Buffer Titration Curve Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer region: Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. Choosing the right biological buffer. Choose a buffer based on your ph requirements. Tris Buffer Titration Curve.
From mungfali.com
Buffer Region On Titration Curve Tris Buffer Titration Curve Integrated heat plots for titration of tris (a) and tes (b) with metal ions. With a moderate amount of naoh added, we have a solution that contains. The concentrations of tris and tes buffer were 1. Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give. Tris Buffer Titration Curve.
From www.researchgate.net
UV/Vis absorption spectra of (a) 10a and (b) 10f in TrisHCl buffer Tris Buffer Titration Curve Integrated heat plots for titration of tris (a) and tes (b) with metal ions. The concentrations of tris and tes buffer were 1. With a moderate amount of naoh added, we have a solution that contains. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Buffer titration the following table can be used to determine. Tris Buffer Titration Curve.
From mungfali.com
Titration Graph Tris Buffer Titration Curve With a moderate amount of naoh added, we have a solution that contains. Choose a buffer based on your ph requirements as well as the pka, a measure of acid strength that. Influence of tris buffer on ph and titration curve. Titration curve for the titration of 20 ml of 0.88 m acetic acid with 0.35 m sodium hydroxide buffer. Tris Buffer Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Tris Buffer Titration Curve Buffer titration the following table can be used to determine the appropriate mix of 1.0 m tris and 1.0 m hydrochloric acid to give the desired ph. The concentrations of tris and tes buffer were 1. Example curve of a titer determination of hydrochloric acid with tris as primary standard. Integrated heat plots for titration of tris (a) and tes. Tris Buffer Titration Curve.