Dilution Problem Formula . See examples of calculating molarity, volume and amount of. Learn how to solve dilution problems in chemistry with this video tutorial. You dilute the solution by adding enough water to. \text{ml}\) of a \(2.0 \: Learn how to dilute a solution by adding solvent without changing the number of moles of solute. See formulas and examples for calculating. Suppose that you have \(100. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to solve dilution problems using the equation m1v1 = m2v2. This chemistry video tutorial explains how to solve common dilution problems using a simple.
from www.educba.com
See formulas and examples for calculating. Learn how to dilute a solution by adding solvent without changing the number of moles of solute. Suppose that you have \(100. \text{ml}\) of a \(2.0 \: See examples of calculating molarity, volume and amount of. Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. You dilute the solution by adding enough water to.
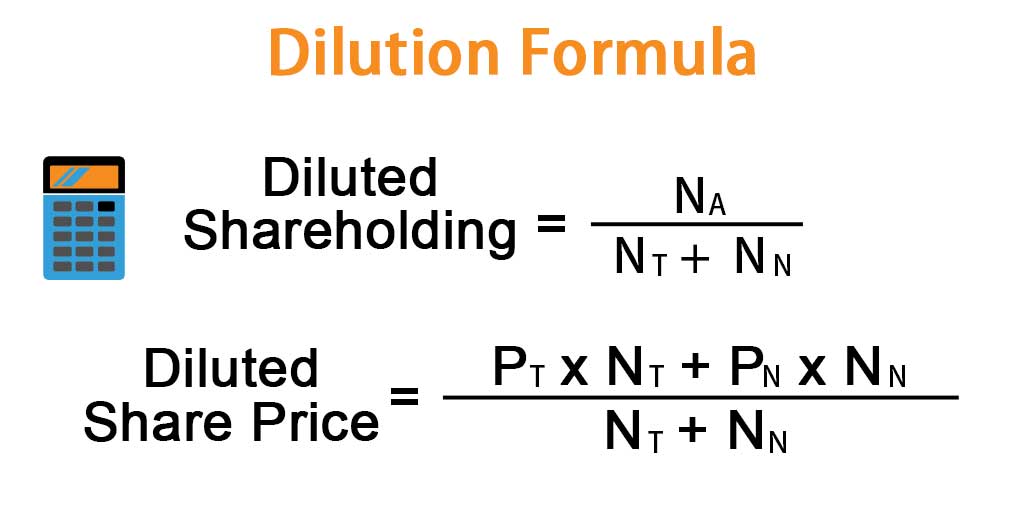
Dilution Formula Calculator (Examples with Excel Template)
Dilution Problem Formula Suppose that you have \(100. This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. See formulas and examples for calculating. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. You dilute the solution by adding enough water to. \text{ml}\) of a \(2.0 \: Learn how to dilute a solution by adding solvent without changing the number of moles of solute. Suppose that you have \(100. See examples of calculating molarity, volume and amount of.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Problem Formula Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to dilute a solution by adding solvent without changing the number of moles of solute. Suppose that you have \(100. Learn how to calculate the concentration and volume of a diluted solution using the. Dilution Problem Formula.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Problem Formula See examples of calculating molarity, volume and amount of. Learn how to dilute a solution by adding solvent without changing the number of moles of solute. This chemistry video tutorial explains how to solve common dilution problems using a simple. \text{ml}\) of a \(2.0 \: Suppose that you have \(100. Learn how to solve dilution problems in chemistry with this. Dilution Problem Formula.
From www.slideserve.com
PPT Study Guide for Dilution PROBLEMS and Concentrations problems Dilution Problem Formula Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn how to solve dilution problems in chemistry with this video tutorial. See examples of calculating molarity, volume and amount of. You dilute the solution by adding enough water to. Suppose that you have \(100.. Dilution Problem Formula.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Problem Formula Learn how to dilute a solution by adding solvent without changing the number of moles of solute. This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume. Dilution Problem Formula.
From cfmuvi.jimdo.com
Simple Serial Dilution Calculation cfmuvi Dilution Problem Formula \text{ml}\) of a \(2.0 \: Learn how to dilute a solution by adding solvent without changing the number of moles of solute. You dilute the solution by adding enough water to. See formulas and examples for calculating. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. Dilution Problem Formula.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Problem Formula Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. You dilute the solution by adding enough water to. See examples. Dilution Problem Formula.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Problem Formula See examples of calculating molarity, volume and amount of. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to solve dilution problems in chemistry with this video tutorial. This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to dilute a solution. Dilution Problem Formula.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Dilution Problem Formula See formulas and examples for calculating. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. This chemistry video tutorial explains how to solve common dilution problems using a simple. Suppose that you have \(100. You dilute the solution by adding enough water to. Learn how to use the dilution equation. Dilution Problem Formula.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Problem Formula This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration. Dilution Problem Formula.
From giordqjfg.blob.core.windows.net
Dilution Problem In Chemistry at Courtney Childress blog Dilution Problem Formula You dilute the solution by adding enough water to. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Suppose that you have \(100. Learn how to solve dilution problems in chemistry with this video tutorial. \text{ml}\) of a \(2.0 \: See examples of calculating. Dilution Problem Formula.
From www.youtube.com
Dilutions using Dilution Factor Bio25 YouTube Dilution Problem Formula See formulas and examples for calculating. This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. \text{ml}\) of a \(2.0 \: See examples of calculating molarity, volume and amount of. Learn. Dilution Problem Formula.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Dilution Problem Formula See examples of calculating molarity, volume and amount of. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Suppose that you have \(100. Learn how to solve dilution problems in chemistry with this video tutorial. \text{ml}\) of a \(2.0 \: Learn how to dilute a solution by adding solvent without. Dilution Problem Formula.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) Dilution Problem Formula See examples of calculating molarity, volume and amount of. You dilute the solution by adding enough water to. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. This chemistry video tutorial explains how to solve common dilution problems using a simple. Suppose that you. Dilution Problem Formula.
From www.slideshare.net
Serial dilution Dilution Problem Formula See examples of calculating molarity, volume and amount of. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to solve dilution problems using. Dilution Problem Formula.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Dilution Problem Formula Suppose that you have \(100. This chemistry video tutorial explains how to solve common dilution problems using a simple. See examples of calculating molarity, volume and amount of. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to solve dilution problems using the equation m1v1 = m2v2. \text{ml}\). Dilution Problem Formula.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Problem Formula Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. See formulas and examples for calculating. See examples of calculating molarity, volume and amount of. \text{ml}\) of a \(2.0 \: Learn how to. Dilution Problem Formula.
From slideplayer.com
Solution Concentration ppt download Dilution Problem Formula Learn how to solve dilution problems in chemistry with this video tutorial. You dilute the solution by adding enough water to. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. See formulas and examples for calculating. Learn how to calculate the concentration and volume. Dilution Problem Formula.
From www.vrogue.co
Bacterial Serial Dilution Method Centuryclever vrogue.co Dilution Problem Formula Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Suppose that you have \(100. See examples of calculating molarity, volume and amount of. This chemistry video tutorial explains how to solve common dilution problems using a simple. You dilute the solution by adding enough. Dilution Problem Formula.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Problem Formula This chemistry video tutorial explains how to solve common dilution problems using a simple. See examples of calculating molarity, volume and amount of. Suppose that you have \(100. See formulas and examples for calculating. Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to solve dilution problems in chemistry with this video tutorial. Learn how. Dilution Problem Formula.
From www.youtube.com
How solve dilution and concentration calculation problems YouTube Dilution Problem Formula Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to solve dilution problems using the equation m1v1 = m2v2. See formulas and examples for calculating. See examples of calculating molarity, volume and amount of. This chemistry video tutorial explains how to solve common dilution problems using a simple. Suppose that you have \(100. You dilute. Dilution Problem Formula.
From www.youtube.com
solution to primer stock dilutions problem sets YouTube Dilution Problem Formula Suppose that you have \(100. You dilute the solution by adding enough water to. This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. See formulas and examples for calculating. \text{ml}\). Dilution Problem Formula.
From studylib.net
Key DilutionsWorksheet Dilution Problem Formula See formulas and examples for calculating. This chemistry video tutorial explains how to solve common dilution problems using a simple. Suppose that you have \(100. Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. See examples of calculating. Dilution Problem Formula.
From giordqjfg.blob.core.windows.net
Dilution Problem In Chemistry at Courtney Childress blog Dilution Problem Formula See formulas and examples for calculating. Learn how to dilute a solution by adding solvent without changing the number of moles of solute. Learn how to solve dilution problems using the equation m1v1 = m2v2. Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to use the dilution equation m1v1 = m2v2 to calculate the. Dilution Problem Formula.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Problem Formula Learn how to solve dilution problems using the equation m1v1 = m2v2. Suppose that you have \(100. Learn how to dilute a solution by adding solvent without changing the number of moles of solute. \text{ml}\) of a \(2.0 \: Learn how to solve dilution problems in chemistry with this video tutorial. You dilute the solution by adding enough water to.. Dilution Problem Formula.
From www.youtube.com
Dilutions Explained with Problems YouTube Dilution Problem Formula Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. You dilute the solution by adding enough water to. Learn how to solve dilution problems in. Dilution Problem Formula.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Problem Formula Learn how to solve dilution problems using the equation m1v1 = m2v2. See formulas and examples for calculating. This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation. Dilution Problem Formula.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Problem Formula Learn how to solve dilution problems in chemistry with this video tutorial. See formulas and examples for calculating. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn how to dilute a solution by adding solvent without changing the number of moles of solute.. Dilution Problem Formula.
From giordqjfg.blob.core.windows.net
Dilution Problem In Chemistry at Courtney Childress blog Dilution Problem Formula Learn how to dilute a solution by adding solvent without changing the number of moles of solute. Learn how to solve dilution problems in chemistry with this video tutorial. See formulas and examples for calculating. This chemistry video tutorial explains how to solve common dilution problems using a simple. See examples of calculating molarity, volume and amount of. Learn how. Dilution Problem Formula.
From lessoncampusunspelt.z13.web.core.windows.net
Molarity By Dilution Worksheet Dilution Problem Formula Learn how to solve dilution problems using the equation m1v1 = m2v2. This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. Learn how to dilute a solution by adding solvent without changing the number of moles. Dilution Problem Formula.
From www.youtube.com
Calculating Dilutions C1V1=C2V2 PowerPoint YouTube Dilution Problem Formula See formulas and examples for calculating. Learn how to solve dilution problems in chemistry with this video tutorial. See examples of calculating molarity, volume and amount of. This chemistry video tutorial explains how to solve common dilution problems using a simple. You dilute the solution by adding enough water to. Suppose that you have \(100. Learn how to solve dilution. Dilution Problem Formula.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Problem Formula This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to solve dilution problems using the equation. Dilution Problem Formula.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Problem Formula This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to solve dilution problems in chemistry with this video tutorial. See formulas and examples for calculating. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and indicator words. \text{ml}\) of a \(2.0 \: Suppose that you have. Dilution Problem Formula.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Problem Formula \text{ml}\) of a \(2.0 \: Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or removing solvent. See examples of calculating molarity, volume and amount of. Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to solve dilution problems using the equation. Dilution Problem Formula.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Problem Formula Learn how to dilute a solution by adding solvent without changing the number of moles of solute. You dilute the solution by adding enough water to. This chemistry video tutorial explains how to solve common dilution problems using a simple. Learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution. Dilution Problem Formula.
From giordqjfg.blob.core.windows.net
Dilution Problem In Chemistry at Courtney Childress blog Dilution Problem Formula You dilute the solution by adding enough water to. Learn how to dilute a solution by adding solvent without changing the number of moles of solute. Suppose that you have \(100. Learn how to solve dilution problems in chemistry with this video tutorial. Learn how to calculate the concentration and volume of a diluted solution using the dilution equation and. Dilution Problem Formula.