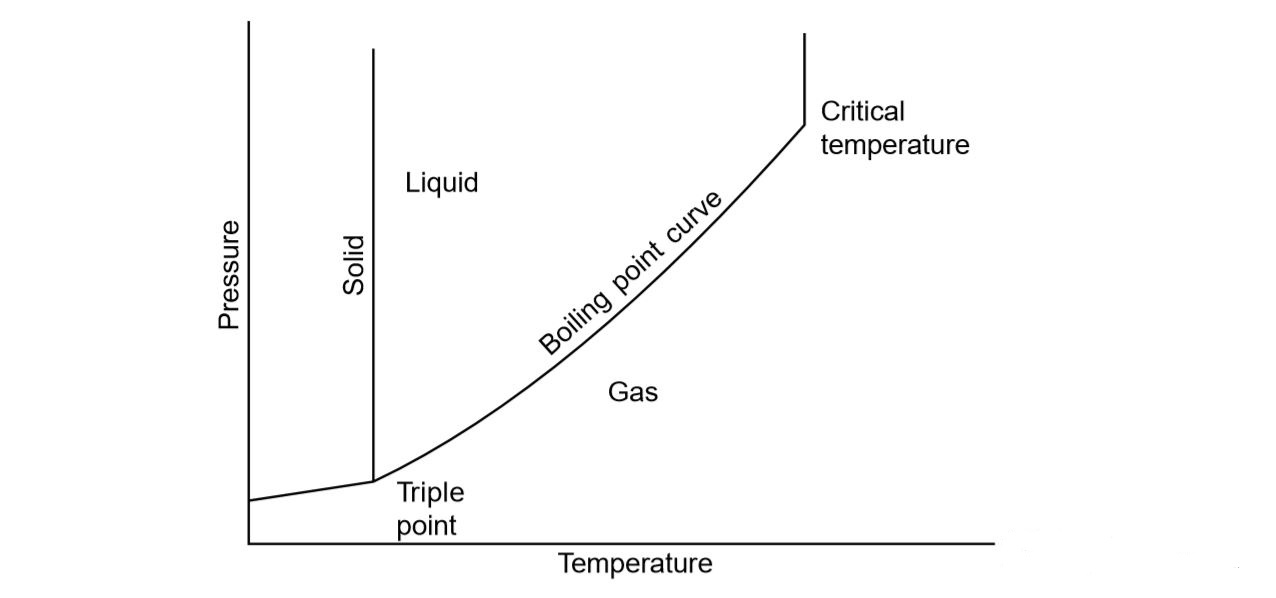
Why Is Water S Boiling Point So High . This increases the effective size of water molecules too, which in turn increases the van der waals. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. So despite its small molecular weight, water has an incredibly big boiling point. The stronger the intermolecular forces, the higher the boiling point. Find out the normal and standard boiling points of water and other liquids, and. This is because water requires more energy to break its. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid.
from engineeringstuff.co.in
Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. Find out the normal and standard boiling points of water and other liquids, and. So despite its small molecular weight, water has an incredibly big boiling point. This is because water requires more energy to break its. The stronger the intermolecular forces, the higher the boiling point. This increases the effective size of water molecules too, which in turn increases the van der waals. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. A water molecule can form a maximum of $4$ $\ce{h}$ bonds.
What is Boiling point Engineeringstuff
Why Is Water S Boiling Point So High This increases the effective size of water molecules too, which in turn increases the van der waals. This increases the effective size of water molecules too, which in turn increases the van der waals. Find out the normal and standard boiling points of water and other liquids, and. This is because water requires more energy to break its. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. The stronger the intermolecular forces, the higher the boiling point. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. So despite its small molecular weight, water has an incredibly big boiling point. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Why Is Water S Boiling Point So High A water molecule can form a maximum of $4$ $\ce{h}$ bonds. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. This is because water requires more energy to break its. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. So despite its small molecular weight, water. Why Is Water S Boiling Point So High.
From chem.libretexts.org
10.4 Properties of Liquids Chemistry LibreTexts Why Is Water S Boiling Point So High Find out the normal and standard boiling points of water and other liquids, and. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of. Why Is Water S Boiling Point So High.
From engineerexcel.com
Boiling Point of Water at Different Pressures EngineerExcel Why Is Water S Boiling Point So High So despite its small molecular weight, water has an incredibly big boiling point. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Learn. Why Is Water S Boiling Point So High.
From gioqefbqp.blob.core.windows.net
Why Isn't My Hot Tub Filling Up With Water at Robin Little blog Why Is Water S Boiling Point So High This increases the effective size of water molecules too, which in turn increases the van der waals. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. This is because water requires more energy to break its. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. Two oxygen. Why Is Water S Boiling Point So High.
From www.animalia-life.club
Boiling Point Of Water For Kids Why Is Water S Boiling Point So High A water molecule can form a maximum of $4$ $\ce{h}$ bonds. This is because water requires more energy to break its. The stronger the intermolecular forces, the higher the boiling point. Find out the normal and standard boiling points of water and other liquids, and. This increases the effective size of water molecules too, which in turn increases the van. Why Is Water S Boiling Point So High.
From snipe.fm
😍 How does salt affect boiling point. Why Does Adding Salt Increase the Why Is Water S Boiling Point So High So despite its small molecular weight, water has an incredibly big boiling point. This increases the effective size of water molecules too, which in turn increases the van der waals. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. Find out the normal and standard boiling points of water and other liquids,. Why Is Water S Boiling Point So High.
From www.chefstemp.com
How to calibrate a thermometer in boiling water ChefsTemp Why Is Water S Boiling Point So High A water molecule can form a maximum of $4$ $\ce{h}$ bonds. The stronger the intermolecular forces, the higher the boiling point. This is because water requires more energy to break its. So despite its small molecular weight, water has an incredibly big boiling point. This increases the effective size of water molecules too, which in turn increases the van der. Why Is Water S Boiling Point So High.
From ar.inspiredpencil.com
Boiling Point Of Water Examples Why Is Water S Boiling Point So High A water molecule can form a maximum of $4$ $\ce{h}$ bonds. The stronger the intermolecular forces, the higher the boiling point. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. This increases the effective size of water molecules too, which in turn increases the van der waals. Find out. Why Is Water S Boiling Point So High.
From ar.inspiredpencil.com
Boiling Point Of Water Examples Why Is Water S Boiling Point So High The stronger the intermolecular forces, the higher the boiling point. This increases the effective size of water molecules too, which in turn increases the van der waals. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. This. Why Is Water S Boiling Point So High.
From yesikame.blogspot.com
Temperature Of Boiling Water / Proc Tech & Oper Acad Sensible & Latent Why Is Water S Boiling Point So High This is because water requires more energy to break its. So despite its small molecular weight, water has an incredibly big boiling point. The stronger the intermolecular forces, the higher the boiling point. Find out the normal and standard boiling points of water and other liquids, and. Two oxygen molecules are attracted to each other through london dispersion forces (induced. Why Is Water S Boiling Point So High.
From mavink.com
Elevation In Boiling Point Graph Why Is Water S Boiling Point So High A water molecule can form a maximum of $4$ $\ce{h}$ bonds. The stronger the intermolecular forces, the higher the boiling point. This is because water requires more energy to break its. So despite its small molecular weight, water has an incredibly big boiling point. Find out the normal and standard boiling points of water and other liquids, and. Two oxygen. Why Is Water S Boiling Point So High.
From www.youtube.com
Boiling point of oil is greater than water ? YouTube Why Is Water S Boiling Point So High This is because water requires more energy to break its. So despite its small molecular weight, water has an incredibly big boiling point. Find out the normal and standard boiling points of water and other liquids, and. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. Learn how water's unusual and unique properties are related to its hydrogen. Why Is Water S Boiling Point So High.
From fyojdchgy.blob.core.windows.net
Deionised Water Boiling Point at Sherry Green blog Why Is Water S Boiling Point So High Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. The stronger the intermolecular forces, the higher the boiling point. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. This increases the effective size of water. Why Is Water S Boiling Point So High.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Why Is Water S Boiling Point So High Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. Find out the normal and standard boiling points of water and other liquids, and. This is because water requires more energy to break its. This increases the effective size of water molecules too, which in turn increases the van der waals. Two oxygen. Why Is Water S Boiling Point So High.
From askfilo.com
The Boiling point of water at normal atmospheric pressure is Filo Why Is Water S Boiling Point So High Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. So despite its small molecular weight, water has an incredibly big boiling point. A water molecule can form a maximum of $4$ $\ce{h}$. Why Is Water S Boiling Point So High.
From gioqefbqp.blob.core.windows.net
Why Isn't My Hot Tub Filling Up With Water at Robin Little blog Why Is Water S Boiling Point So High Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Find out the normal and standard boiling points of water and other liquids, and. This is because water requires more energy to break its. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. Learn how the boiling. Why Is Water S Boiling Point So High.
From www.myxxgirl.com
Boiling Point Of Water Chart My XXX Hot Girl Why Is Water S Boiling Point So High Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. This increases the effective size of water molecules too, which in turn increases the van der waals. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. The stronger the intermolecular forces, the. Why Is Water S Boiling Point So High.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes Why Is Water S Boiling Point So High Find out the normal and standard boiling points of water and other liquids, and. The stronger the intermolecular forces, the higher the boiling point. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. This increases the effective size of water molecules too, which in turn increases the van der. Why Is Water S Boiling Point So High.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Why Is Water S Boiling Point So High So despite its small molecular weight, water has an incredibly big boiling point. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Learn how water's unusual and unique properties are related to its hydrogen. Why Is Water S Boiling Point So High.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Why Is Water S Boiling Point So High This increases the effective size of water molecules too, which in turn increases the van der waals. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. This is because water requires more energy to break its. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. A. Why Is Water S Boiling Point So High.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox Why Is Water S Boiling Point So High This is because water requires more energy to break its. The stronger the intermolecular forces, the higher the boiling point. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. A water molecule can form. Why Is Water S Boiling Point So High.
From sciencenotes.org
How to Boil Water at Room Temperature Why Is Water S Boiling Point So High Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. This is because water requires more energy to break its. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and. Why Is Water S Boiling Point So High.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point Why Is Water S Boiling Point So High This increases the effective size of water molecules too, which in turn increases the van der waals. This is because water requires more energy to break its. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. Find out the normal and standard boiling points of water and other liquids,. Why Is Water S Boiling Point So High.
From www.tessshebaylo.com
Chemical Equation For Water Boiling Tessshebaylo Why Is Water S Boiling Point So High Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. The stronger the intermolecular forces, the higher the boiling point. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. This is because water requires more energy to break its. Learn how the boiling point of a liquid depends on the surrounding. Why Is Water S Boiling Point So High.
From hxenwilps.blob.core.windows.net
SolidLiquid Phase Change Phenomena at Bobbie ODonnell blog Why Is Water S Boiling Point So High A water molecule can form a maximum of $4$ $\ce{h}$ bonds. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. The stronger the intermolecular forces, the higher the boiling point. This increases. Why Is Water S Boiling Point So High.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country Why Is Water S Boiling Point So High This increases the effective size of water molecules too, which in turn increases the van der waals. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. This is because water requires more energy to break its. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. Find out. Why Is Water S Boiling Point So High.
From www.youtube.com
Why is the Boiling Point of water (H2O) so high? YouTube Why Is Water S Boiling Point So High The stronger the intermolecular forces, the higher the boiling point. This increases the effective size of water molecules too, which in turn increases the van der waals. Find out the normal and standard boiling points of water and other liquids, and. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the. Why Is Water S Boiling Point So High.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Why Is Water S Boiling Point So High A water molecule can form a maximum of $4$ $\ce{h}$ bonds. This increases the effective size of water molecules too, which in turn increases the van der waals. Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. This is because water requires more energy to break its. The stronger. Why Is Water S Boiling Point So High.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Why Is Water S Boiling Point So High A water molecule can form a maximum of $4$ $\ce{h}$ bonds. This is because water requires more energy to break its. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Find out the normal and standard boiling points of water and other liquids, and. So despite its small molecular weight, water has an incredibly. Why Is Water S Boiling Point So High.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff Why Is Water S Boiling Point So High Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. The stronger the intermolecular forces, the higher the boiling point. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. So despite its small molecular weight, water has an incredibly big boiling point. This is. Why Is Water S Boiling Point So High.
From www.perkinselearning.org
Can the Boiling Point of Water be Changed? Perkins eLearning Why Is Water S Boiling Point So High The stronger the intermolecular forces, the higher the boiling point. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. This is because water requires more energy to break its. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. This increases the effective size of water molecules. Why Is Water S Boiling Point So High.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Why Is Water S Boiling Point So High Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. This is because water requires more energy to break its. Find out the normal and standard boiling points of water and other liquids, and. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. The stronger the intermolecular forces, the higher the boiling point.. Why Is Water S Boiling Point So High.
From vandewaterbooks.com
What Temperature Does Water Boil At? Boiling Point & Elevation Vande Why Is Water S Boiling Point So High Learn how the boiling point of a liquid depends on the surrounding pressure and the vapor pressure of the liquid. The stronger the intermolecular forces, the higher the boiling point. A water molecule can form a maximum of $4$ $\ce{h}$ bonds. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. Find out. Why Is Water S Boiling Point So High.
From hinative.com
Just a quick question, I was reading a story in Italian and encountered Why Is Water S Boiling Point So High So despite its small molecular weight, water has an incredibly big boiling point. The stronger the intermolecular forces, the higher the boiling point. This increases the effective size of water molecules too, which in turn increases the van der waals. Two oxygen molecules are attracted to each other through london dispersion forces (induced temporary dipoles. Learn how the boiling point. Why Is Water S Boiling Point So High.
From www.animalia-life.club
Boiling Point Of Water For Kids Why Is Water S Boiling Point So High A water molecule can form a maximum of $4$ $\ce{h}$ bonds. This increases the effective size of water molecules too, which in turn increases the van der waals. The stronger the intermolecular forces, the higher the boiling point. Learn how water's unusual and unique properties are related to its hydrogen bonding, polarity, and intermolecular forces. So despite its small molecular. Why Is Water S Boiling Point So High.