Bromine Isotopes Percent Abundance . Silver (ag) has two stable isotopes: List, data and properties of all known isotopes of bromine. Bromine has two naturally occurring isotopes: In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. This chemistry video tutorial explains how to find the percent abundance of an isotope. Table of isotopic masses and natural abundances. This table lists the mass and percent natural abundance for the stable nuclides. This is not to be confused with the. All atomic nuclei of the chemical element bromine are summarized under. Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two peaks of 1:1. The mass of the longest lived isotope is given for elements. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\).
from www.numerade.com
List, data and properties of all known isotopes of bromine. Table of isotopic masses and natural abundances. All atomic nuclei of the chemical element bromine are summarized under. Silver (ag) has two stable isotopes: This table lists the mass and percent natural abundance for the stable nuclides. This is not to be confused with the. This chemistry video tutorial explains how to find the percent abundance of an isotope. The mass of the longest lived isotope is given for elements. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer.
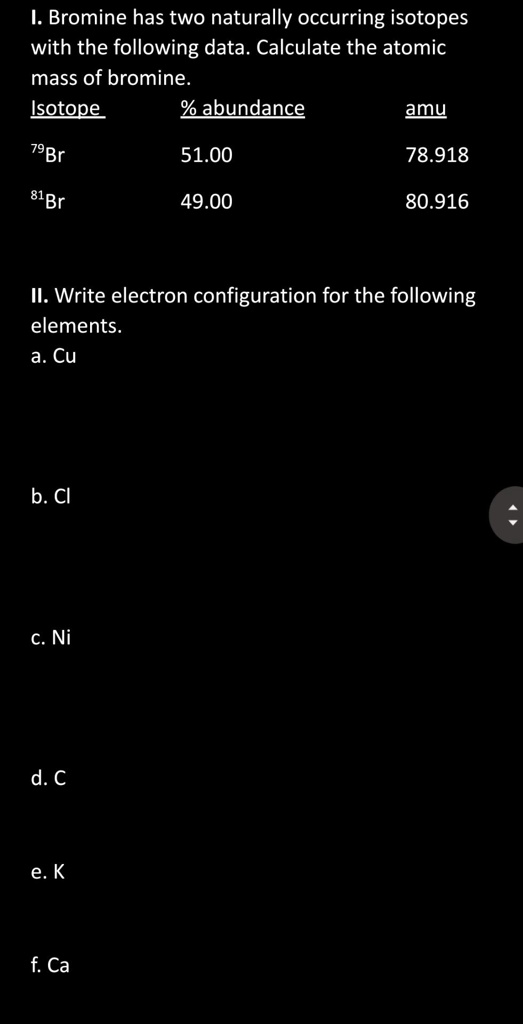
SOLVED I. Bromine has two naturally occurring isotopes with the following data Calculate the
Bromine Isotopes Percent Abundance Silver (ag) has two stable isotopes: This table lists the mass and percent natural abundance for the stable nuclides. List, data and properties of all known isotopes of bromine. The mass of the longest lived isotope is given for elements. Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two peaks of 1:1. All atomic nuclei of the chemical element bromine are summarized under. This is not to be confused with the. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. This chemistry video tutorial explains how to find the percent abundance of an isotope. Silver (ag) has two stable isotopes: Table of isotopic masses and natural abundances. Bromine has two naturally occurring isotopes:
From www.numerade.com
SOLVEDNaturally occurring bromine consists of two isotopes, ^79 Br and ^si Br, with isotopic Bromine Isotopes Percent Abundance This table lists the mass and percent natural abundance for the stable nuclides. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). Silver (ag) has two stable isotopes: All atomic nuclei of the chemical element bromine are summarized under. Table of isotopic masses and natural abundances. Bromine has two naturally occurring isotopes: List, data and properties. Bromine Isotopes Percent Abundance.
From lessonlistelfriede.z19.web.core.windows.net
Calculate Percent Abundance Of 3 Isotopes Bromine Isotopes Percent Abundance All atomic nuclei of the chemical element bromine are summarized under. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). Bromine has two naturally occurring isotopes: List, data and properties of all known isotopes of bromine. This chemistry video tutorial explains how to find the percent abundance of an isotope. Bromine also has two naturally occurring. Bromine Isotopes Percent Abundance.
From www.youtube.com
Finding Percent Abundance (3 Isotopes) YouTube Bromine Isotopes Percent Abundance List, data and properties of all known isotopes of bromine. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). This is not to be confused with the. Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for. Bromine Isotopes Percent Abundance.
From www.youtube.com
How to calculate percentage abundance of each isotope ? YouTube Bromine Isotopes Percent Abundance The mass of the longest lived isotope is given for elements. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). All atomic nuclei of the chemical element bromine are summarized under. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. This chemistry video. Bromine Isotopes Percent Abundance.
From imgflip.com
Bromine Isotopic Abundance Imgflip Bromine Isotopes Percent Abundance This table lists the mass and percent natural abundance for the stable nuclides. Table of isotopic masses and natural abundances. Silver (ag) has two stable isotopes: List, data and properties of all known isotopes of bromine. Bromine has two naturally occurring isotopes: This is not to be confused with the. This is observed in the mass spectrum of bromobenzene shown. Bromine Isotopes Percent Abundance.
From www.numerade.com
SOLVEDBromine has two naturally occurring isotopes. One of them, bromine79, has a mass of 78. Bromine Isotopes Percent Abundance In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. The mass of the longest lived isotope is given for elements. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). Silver (ag) has two stable isotopes: Bromine has two naturally occurring isotopes: All atomic. Bromine Isotopes Percent Abundance.
From www.numerade.com
SOLVEDThe element bromine, Br (atomic number 35), has two major isotopes of similar abundance Bromine Isotopes Percent Abundance In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. List, data and properties of all known isotopes of bromine. Table of isotopic masses and natural abundances. Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of. Bromine Isotopes Percent Abundance.
From byjus.com
what is abundance and how to find abundance of isotopes Bromine Isotopes Percent Abundance List, data and properties of all known isotopes of bromine. This table lists the mass and percent natural abundance for the stable nuclides. The mass of the longest lived isotope is given for elements. Bromine has two naturally occurring isotopes: Silver (ag) has two stable isotopes: Table of isotopic masses and natural abundances. Bromine also has two naturally occurring isotopes,. Bromine Isotopes Percent Abundance.
From www.youtube.com
Calculating Isotope Abundance using Atomic Mass YouTube Bromine Isotopes Percent Abundance The mass of the longest lived isotope is given for elements. This is not to be confused with the. This table lists the mass and percent natural abundance for the stable nuclides. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. Table of isotopic masses and natural. Bromine Isotopes Percent Abundance.
From chem.libretexts.org
6.4 Isotope Abundance Chemistry LibreTexts Bromine Isotopes Percent Abundance The mass of the longest lived isotope is given for elements. List, data and properties of all known isotopes of bromine. Silver (ag) has two stable isotopes: This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). This chemistry video tutorial explains how to find the percent abundance of an isotope. Bromine also has two naturally occurring. Bromine Isotopes Percent Abundance.
From masterconceptsinchemistry.com
How to calculate mass of an isotope from isotopic mass and percentage abundance Bromine Isotopes Percent Abundance This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two peaks of 1:1. List, data and properties of all known isotopes of bromine. All atomic nuclei. Bromine Isotopes Percent Abundance.
From www.numerade.com
SOLVEDThe mass spectrum of bromine, Br2, is shown in Figure P2.102. The natural abundances of Bromine Isotopes Percent Abundance This is not to be confused with the. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). The mass of the longest lived isotope is given for elements. Bromine has two naturally occurring isotopes: Silver (ag) has two stable isotopes: In the above, the most intense ion is set to 100% since this corresponds best to. Bromine Isotopes Percent Abundance.
From www.numerade.com
SOLVEDBromine is a diatomic molecule, and it has two isotopes, ^79 Br and ^81 Br, with a Bromine Isotopes Percent Abundance Silver (ag) has two stable isotopes: This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two peaks of 1:1. This chemistry video tutorial explains how to. Bromine Isotopes Percent Abundance.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Isotopes Percent Abundance Silver (ag) has two stable isotopes: All atomic nuclei of the chemical element bromine are summarized under. Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two peaks of 1:1. This is observed in the mass spectrum of bromobenzene. Bromine Isotopes Percent Abundance.
From www.numerade.com
SOLVED I. Bromine has two naturally occurring isotopes with the following data Calculate the Bromine Isotopes Percent Abundance All atomic nuclei of the chemical element bromine are summarized under. The mass of the longest lived isotope is given for elements. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). List, data and properties of all known isotopes of bromine. Bromine has two naturally occurring isotopes: This is not to be confused with the. This. Bromine Isotopes Percent Abundance.
From www.numerade.com
Bromine has two naturally occurring isotopes (Br79 and Br81) and an atomic mass of 79.904 amu Bromine Isotopes Percent Abundance Silver (ag) has two stable isotopes: This chemistry video tutorial explains how to find the percent abundance of an isotope. List, data and properties of all known isotopes of bromine. The mass of the longest lived isotope is given for elements. Bromine has two naturally occurring isotopes: Table of isotopic masses and natural abundances. This is observed in the mass. Bromine Isotopes Percent Abundance.
From www.numerade.com
SOLVEDCalculate the atomic mass of bromine, given the following information about bromine's Bromine Isotopes Percent Abundance This table lists the mass and percent natural abundance for the stable nuclides. Bromine has two naturally occurring isotopes: In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). Silver (ag) has two stable isotopes:. Bromine Isotopes Percent Abundance.
From slideplayer.com
Chapter 4 Atomic Structure. ppt download Bromine Isotopes Percent Abundance Bromine has two naturally occurring isotopes: The mass of the longest lived isotope is given for elements. This chemistry video tutorial explains how to find the percent abundance of an isotope. This table lists the mass and percent natural abundance for the stable nuclides. All atomic nuclei of the chemical element bromine are summarized under. This is observed in the. Bromine Isotopes Percent Abundance.
From www.researchgate.net
Patterns of the molecular ion with multiple chlorine or bromine atoms.... Download Scientific Bromine Isotopes Percent Abundance This is not to be confused with the. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). Silver (ag) has two stable isotopes: Bromine has two naturally occurring isotopes: This table lists the mass. Bromine Isotopes Percent Abundance.
From www.youtube.com
Calculating Percentage abundance of each isotope YouTube Bromine Isotopes Percent Abundance List, data and properties of all known isotopes of bromine. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). This is not to be confused with the. This table lists the mass and percent natural abundance for the stable nuclides. Bromine has two naturally occurring isotopes: Silver (ag) has two stable isotopes: In the above, the. Bromine Isotopes Percent Abundance.
From www.numerade.com
SOLVED QUESTION 8 point= QUESTION Bromine has two isotopes, 79Br and 8Br To find out the Bromine Isotopes Percent Abundance Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two peaks of 1:1. This is not to be confused with the. All atomic nuclei of the chemical element bromine are summarized under. The mass of the longest lived isotope. Bromine Isotopes Percent Abundance.
From www.youtube.com
Atomic Mass How to Calculate Isotope Abundance YouTube Bromine Isotopes Percent Abundance This is not to be confused with the. This chemistry video tutorial explains how to find the percent abundance of an isotope. All atomic nuclei of the chemical element bromine are summarized under. Silver (ag) has two stable isotopes: In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. Bromine Isotopes Percent Abundance.
From www.sliderbase.com
Mass Spectrometry Presentation Chemistry Bromine Isotopes Percent Abundance All atomic nuclei of the chemical element bromine are summarized under. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. Silver (ag) has two stable isotopes: The mass of the longest lived isotope is given for elements. This is not to be confused with the. List, data. Bromine Isotopes Percent Abundance.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine Bromine Isotopes Percent Abundance In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). This table lists the mass and percent natural abundance for the stable nuclides. Table of isotopic masses and natural abundances. Silver (ag) has two stable. Bromine Isotopes Percent Abundance.
From www.transformationtutoring.com
Percent Abundance and Average Atomic Mass Calculations Guide Bromine Isotopes Percent Abundance Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two peaks of 1:1. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). Bromine has two naturally occurring isotopes: Table of isotopic masses and natural abundances.. Bromine Isotopes Percent Abundance.
From www.youtube.com
calculate percent abundance of each isotope YouTube Bromine Isotopes Percent Abundance The mass of the longest lived isotope is given for elements. List, data and properties of all known isotopes of bromine. This table lists the mass and percent natural abundance for the stable nuclides. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). This chemistry video tutorial explains how to find the percent abundance of an. Bromine Isotopes Percent Abundance.
From www.numerade.com
SOLVED Consider bromine, which occur as a mixture of two isotopes bromine79, with natural Bromine Isotopes Percent Abundance Silver (ag) has two stable isotopes: This table lists the mass and percent natural abundance for the stable nuclides. Bromine has two naturally occurring isotopes: List, data and properties of all known isotopes of bromine. Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in. Bromine Isotopes Percent Abundance.
From freeonlinehelpns.blogspot.com
Free Online Help Bromine has two naturally occurring isotopes (and ) and has an atomic mass of Bromine Isotopes Percent Abundance List, data and properties of all known isotopes of bromine. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). This chemistry video tutorial explains how to find the percent abundance of an isotope. Table of isotopic masses and natural abundances. This table lists the mass and percent natural abundance for the stable nuclides. In the above,. Bromine Isotopes Percent Abundance.
From www.shimadzu.com
Isotope Abundance Table SHIMADZU (Shimadzu Corporation) Bromine Isotopes Percent Abundance Bromine has two naturally occurring isotopes: This chemistry video tutorial explains how to find the percent abundance of an isotope. The mass of the longest lived isotope is given for elements. Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for. Bromine Isotopes Percent Abundance.
From www.youtube.com
How to Solve for Percent Abundance of Isotopes Examples, Practice Problems, Step by Step Bromine Isotopes Percent Abundance In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. The mass of the longest lived isotope is given for elements. Table of isotopic masses and natural abundances. Bromine has two naturally occurring isotopes: List, data and properties of all known isotopes of bromine. This is observed in. Bromine Isotopes Percent Abundance.
From www.numerade.com
SOLVED QUESTION Bromine has two isotopes, 79Br and 0Br To find out the isotopic abundance of Bromine Isotopes Percent Abundance This chemistry video tutorial explains how to find the percent abundance of an isotope. Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two peaks of 1:1. All atomic nuclei of the chemical element bromine are summarized under. The. Bromine Isotopes Percent Abundance.
From www.chegg.com
Solved Bromine (Br) has two stable isotopes. Their masses Bromine Isotopes Percent Abundance In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass spectrometer. Table of isotopic masses and natural abundances. Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two. Bromine Isotopes Percent Abundance.
From slideplayer.com
GENERAL CHEMISTRY Chapter 2 Atoms and the Atomic Theory ppt download Bromine Isotopes Percent Abundance This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). Table of isotopic masses and natural abundances. This is not to be confused with the. This table lists the mass and percent natural abundance for the stable nuclides. In the above, the most intense ion is set to 100% since this corresponds best to the output from. Bromine Isotopes Percent Abundance.
From www.ciaaw.org
Atomic Weight of Bromine Commission on Isotopic Abundances and Atomic Weights Bromine Isotopes Percent Abundance The mass of the longest lived isotope is given for elements. All atomic nuclei of the chemical element bromine are summarized under. Silver (ag) has two stable isotopes: Bromine also has two naturally occurring isotopes, 79 br is the most abundant and 81 br has a relative abundance of 98% which results in a relative intensity for these two peaks. Bromine Isotopes Percent Abundance.
From www.youtube.com
13.04 Isotopic Abundance in Mass Spectrometry YouTube Bromine Isotopes Percent Abundance Silver (ag) has two stable isotopes: This table lists the mass and percent natural abundance for the stable nuclides. This is observed in the mass spectrum of bromobenzene shown in figure \(\pageindex{3}\). List, data and properties of all known isotopes of bromine. The mass of the longest lived isotope is given for elements. This is not to be confused with. Bromine Isotopes Percent Abundance.