Cell Therapy Product Development . this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the design of these novel engineered cell types in the decision. Slides outlining the fda regulatory pathway and requirements for cell and. key capabilities in the early stages of development for cell and gene therapy products include: this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. us regulation of cell and gene therapy products : the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being.
from nam.edu
a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. us regulation of cell and gene therapy products : key capabilities in the early stages of development for cell and gene therapy products include: this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the design of these novel engineered cell types in the decision. Slides outlining the fda regulatory pathway and requirements for cell and. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being.
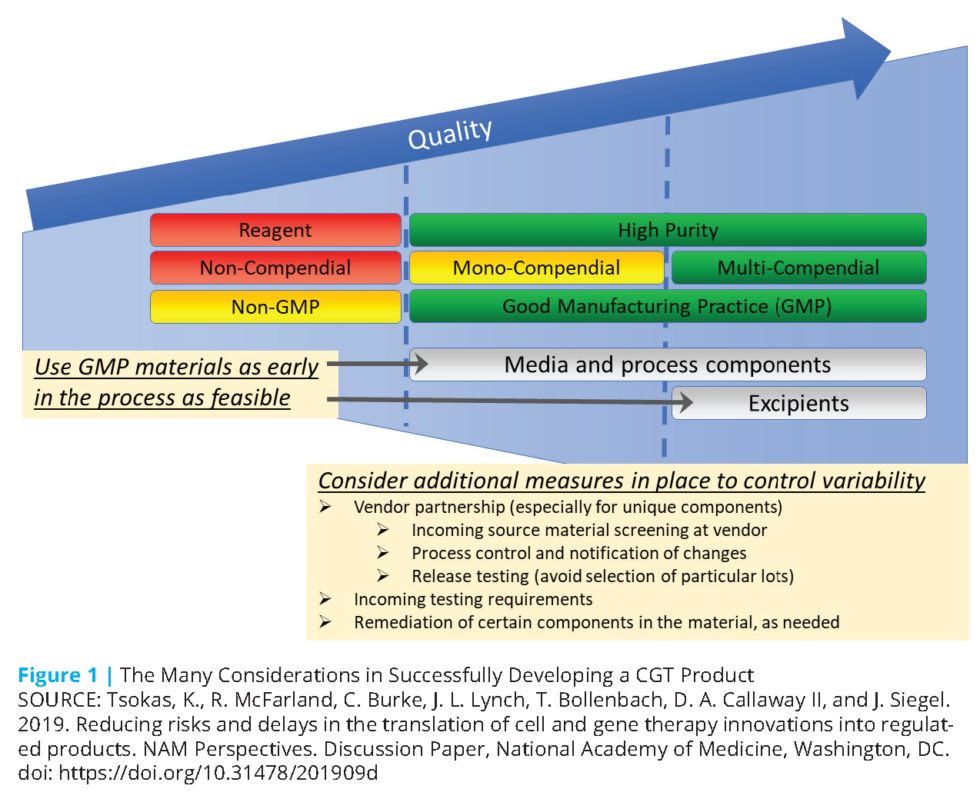
Reducing Risks and Delays in the Translation of Cell and Gene Therapy Innovations into Regulated
Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. the design of these novel engineered cell types in the decision. Slides outlining the fda regulatory pathway and requirements for cell and. key capabilities in the early stages of development for cell and gene therapy products include: this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. us regulation of cell and gene therapy products :
From www.genengnews.com
Confidence in Cell Therapy Development Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. key capabilities in the early stages of development for cell and gene therapy products include: the design of these novel engineered cell types. Cell Therapy Product Development.
From www.phacilitate.com
Allogeneic Therapies Silver Bullet for Developers? Phacilitate Cell Therapy Product Development Slides outlining the fda regulatory pathway and requirements for cell and. the design of these novel engineered cell types in the decision. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. . Cell Therapy Product Development.
From www.jwtherapeutics.com
Process Development JW Therapeutics Cell Therapy Product Development a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. us regulation. Cell Therapy Product Development.
From scienceblog.com
Speeding cell, gene therapy development Cell Therapy Product Development Slides outlining the fda regulatory pathway and requirements for cell and. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. a successful cell therapy will be a consistent, safe, and effective cell product,. Cell Therapy Product Development.
From www.genedata.com
自己由来および同種他家由来の細胞療法への期待と課題 Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. key capabilities in the early stages of development for cell and gene therapy products include: a successful cell therapy will be a. Cell Therapy Product Development.
From www.researchgate.net
(A) Framework of cell therapy product (CTP) development and... Download Scientific Diagram Cell Therapy Product Development a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. key capabilities in the early stages of development for cell and gene therapy products include: us regulation of cell and gene therapy products : the design of these novel engineered cell types in the decision. Slides. Cell Therapy Product Development.
From noblelifesci.com
Navigating Cell Therapy Product Development inar Event Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. key capabilities in the early stages of development for cell and gene therapy products include: a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. the design of these. Cell Therapy Product Development.
From nam.edu
Manufacturing Cell Therapies The Paradigm Shift in Health Care of This Century National Cell Therapy Product Development Slides outlining the fda regulatory pathway and requirements for cell and. key capabilities in the early stages of development for cell and gene therapy products include: the design of these novel engineered cell types in the decision. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. this article. Cell Therapy Product Development.
From www.ppd.com
New and emerging cell and gene therapy development considerations Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. Slides outlining the fda regulatory pathway and requirements for cell and. the uniqueness of cell therapy drug product (dp) originates from the active. Cell Therapy Product Development.
From www.futuretech.org.tw
Systemic Safety Cell Therapy Product Development Low Risk in Tumor Promotion for Allogeneic Cell Therapy Product Development us regulation of cell and gene therapy products : the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. key capabilities in the early stages of development for cell and gene therapy products include: this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles. Cell Therapy Product Development.
From nam.edu
Reducing Risks and Delays in the Translation of Cell and Gene Therapy Innovations into Regulated Cell Therapy Product Development the design of these novel engineered cell types in the decision. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. Slides outlining the fda regulatory pathway and requirements for cell and. us. Cell Therapy Product Development.
From allcells.com
Why You Should Start Clinical Grade Cell Supply Discussions Early in Cell and Gene Therapy Cell Therapy Product Development Slides outlining the fda regulatory pathway and requirements for cell and. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. us regulation of cell and gene therapy products : a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application.. Cell Therapy Product Development.
From www.researchgate.net
(PDF) Selecting a Cell Engineering Methodology During Cell Therapy Product Development Cell Therapy Product Development the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. us regulation of cell and gene therapy products : this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the design of these novel engineered cell types in the decision. Slides outlining the. Cell Therapy Product Development.
From www.cytivalifesciences.com
Cell and gene therapy CDMO role Cytiva Cell Therapy Product Development a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. Slides outlining the fda regulatory pathway and requirements for cell and. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. us regulation of cell and gene therapy products :. Cell Therapy Product Development.
From www.cell.com
Global Manufacturing of CAR T Cell Therapy Molecular Therapy Methods & Clinical Development Cell Therapy Product Development key capabilities in the early stages of development for cell and gene therapy products include: the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. the design of these novel engineered cell types in the decision. us regulation of cell and gene therapy products : this article overviews the. Cell Therapy Product Development.
From opisresearch.com
Advanced Therapy Medicinal Products Opis Europe Cell Therapy Product Development us regulation of cell and gene therapy products : Slides outlining the fda regulatory pathway and requirements for cell and. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. the design of these novel engineered cell types in the decision. key capabilities in the early. Cell Therapy Product Development.
From nam.edu
Manufacturing Cell Therapies The Paradigm Shift in Health Care of This Century National Cell Therapy Product Development the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. key capabilities in the early stages of development for cell and gene therapy products include: the design of these novel engineered cell types. Cell Therapy Product Development.
From www.technologynetworks.com
Regulatory requirements in the development of advanced therapy products (cell, gene therapy Cell Therapy Product Development us regulation of cell and gene therapy products : this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. key capabilities in the early stages of development for cell and gene therapy products include: the design of these novel engineered cell types in the decision. Slides outlining the fda. Cell Therapy Product Development.
From www.roslinct.com
Cell Therapy Analytical Development Lykan Bioscience Cell Therapy Product Development the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. us regulation of cell and gene therapy products : the design of these novel engineered cell types in the decision. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. key capabilities. Cell Therapy Product Development.
From www.miltenyibiotec.com
Cell and gene therapy solutions Miltenyi Biotec USA Cell Therapy Product Development us regulation of cell and gene therapy products : this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. Slides outlining the fda regulatory pathway and requirements for cell and. key capabilities in the early stages of development for cell and gene therapy products include: this article overviews the. Cell Therapy Product Development.
From www.isct-cytotherapy.org
A riskbased approach for cell line development, manufacturing and characterization of Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. the design of these novel engineered cell types in the decision. key capabilities in the early stages of development for cell and gene. Cell Therapy Product Development.
From xtalks.com
Cell & Gene Therapy Product Development How Can You Build a Successful Partnership with Your Cell Therapy Product Development a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. the design of these novel engineered cell types in the decision. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. this article overviews the key technical aspects of. Cell Therapy Product Development.
From www.semanticscholar.org
Figure 2 from Selecting a Cell Engineering Methodology During Cell Therapy Product Development Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. key capabilities. Cell Therapy Product Development.
From nancy-rubin.com
Understanding Cell and Gene Therapy Product Development nancyrubin Cell Therapy Product Development key capabilities in the early stages of development for cell and gene therapy products include: Slides outlining the fda regulatory pathway and requirements for cell and. us regulation of cell and gene therapy products : the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. this article overviews the key. Cell Therapy Product Development.
From voisinconsulting.com
Critical Materials considerations for Cell and Gene therapy product development Part 1 Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. key capabilities in the early stages of development for cell and gene therapy products include: us regulation of cell and gene therapy products : this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental. Cell Therapy Product Development.
From www.engineering.org.cn
Engineered T Cell Therapies from a Drug Development Viewpoint Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. us regulation. Cell Therapy Product Development.
From www.bdbiosciences.com
Cell Therapy Research and Manufacturing QC Tools Cell Therapy Product Development us regulation of cell and gene therapy products : key capabilities in the early stages of development for cell and gene therapy products include: Slides outlining the fda regulatory pathway and requirements for cell and. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the design of these. Cell Therapy Product Development.
From www.pharmexec.com
Operations Gateway to Success in Cell and Gene Therapies Cell Therapy Product Development us regulation of cell and gene therapy products : Slides outlining the fda regulatory pathway and requirements for cell and. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. key capabilities in the early stages of development for cell and gene therapy products include: the. Cell Therapy Product Development.
From bioinsights.azurewebsites.net
BioInsights Toward an integrated model of product characterization for CART cell therapy drug Cell Therapy Product Development a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the design of these novel engineered cell types in the decision. Slides outlining the fda regulatory pathway and requirements for. Cell Therapy Product Development.
From www.semanticscholar.org
Figure 1 from Selecting a Cell Engineering Methodology During Cell Therapy Product Development Cell Therapy Product Development us regulation of cell and gene therapy products : this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. the design of these novel engineered cell types in the decision. Slides outlining. Cell Therapy Product Development.
From www.thermofisher.com
Enabling development of personalized cell therapies Cell Therapy Product Development Slides outlining the fda regulatory pathway and requirements for cell and. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. us regulation of cell and gene therapy products : this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of.. Cell Therapy Product Development.
From www.cytivalifesciences.com
Biopharma process development introduction Cytiva Cell Therapy Product Development this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. key capabilities in the early stages of development for cell and gene therapy products include: Slides outlining the fda regulatory. Cell Therapy Product Development.
From www.slideserve.com
PPT Cell Therapy A Manufacturing Viewpoint PowerPoint Presentation ID294973 Cell Therapy Product Development key capabilities in the early stages of development for cell and gene therapy products include: the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. us regulation of cell and gene therapy products : Slides outlining the fda regulatory pathway and requirements for cell and. this article overviews the key. Cell Therapy Product Development.
From www.criver.com
Derisking Cell Therapy Product Development Charles River Cell Therapy Product Development the uniqueness of cell therapy drug product (dp) originates from the active pharmaceutical ingredient (api) being. the design of these novel engineered cell types in the decision. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. Slides outlining the fda regulatory pathway and requirements for cell. Cell Therapy Product Development.
From www.genengnews.com
Boosting Cell Therapy Production Cell Therapy Product Development key capabilities in the early stages of development for cell and gene therapy products include: this article overviews the key technical aspects of cell therapy dp development and elucidates fundamental principles of. a successful cell therapy will be a consistent, safe, and effective cell product, regardless of the cell type or application. us regulation of cell. Cell Therapy Product Development.