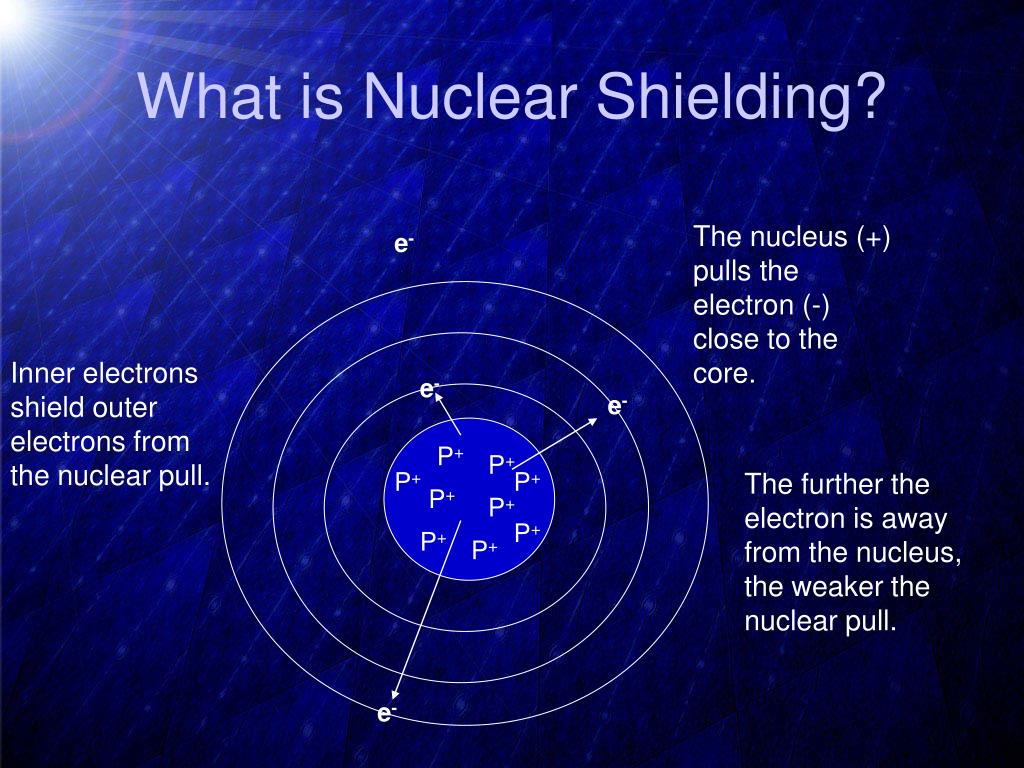
Electron Shielding Down A Group . electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Complete electron shells shield the. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding increases down a group because the nuclear core is farther removed from the valence electrons. Along the period from left to right along a.
from www.slideserve.com
evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. Along the period from left to right along a. Complete electron shells shield the. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free
Electron Shielding Down A Group the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Complete electron shells shield the. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Along the period from left to right along a. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of.
From byjus.com
What is shielding and deshielding in NMR? Give an example? Electron Shielding Down A Group shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. evaluate how. Electron Shielding Down A Group.
From www.chem.fsu.edu
Electron Configurations Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. shielding increases down a group because the nuclear core is farther removed from the valence electrons. Along the period from left to right along a. Complete electron shells shield the. the concept of the shielding effect explains the. Electron Shielding Down A Group.
From slideplayer.com
Periodic Trends. ppt download Electron Shielding Down A Group electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. Complete electron shells shield the. shielding increases down a group because the nuclear core is farther removed from the valence electrons. electron affinity generally decreases down a group of elements because each atom is larger than the atom. Electron Shielding Down A Group.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Electron Shielding Down A Group electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Along the period from left to right along a. the concept of the. Electron Shielding Down A Group.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding Electron Shielding Down A Group Along the period from left to right along a. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. Complete electron shells shield the. evaluate how electron shielding contributes to periodic trends such. Electron Shielding Down A Group.
From www.youtube.com
Electron shielding YouTube Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. shielding increases down a group because the nuclear core. Electron Shielding Down A Group.
From slideplayer.com
Chemsheets AS006 (Electron arrangement) ppt download Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Along the period from left to right along a. shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of electron shielding, in which. Electron Shielding Down A Group.
From slideplayer.com
PERIODIC TABLE REVIEW 10/23/12 CAIAFA. ppt download Electron Shielding Down A Group the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. Along the period from left to right along a. evaluate how electron shielding contributes to periodic trends such as. Electron Shielding Down A Group.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube Electron Shielding Down A Group the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a. Electron Shielding Down A Group.
From socratic.org
How are shielding effect and atomic radius related? Socratic Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. evaluate how electron shielding contributes to periodic trends such as atomic radius and. Electron Shielding Down A Group.
From www.slideserve.com
PPT Unit 5 The Periodic Table PowerPoint Presentation, free download Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. Along the period from left to right along a. electron affinity generally decreases down a group of elements because each atom is larger. Electron Shielding Down A Group.
From slidetodoc.com
Trends in the Periodic Table IDENTIFYING THE PATTERNS Electron Shielding Down A Group shielding increases down a group because the nuclear core is farther removed from the valence electrons. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. . Electron Shielding Down A Group.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID6535293 Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. shielding increases down a group because the nuclear core is farther removed from the valence electrons. Complete electron shells shield the. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced. Electron Shielding Down A Group.
From www.youtube.com
Zeff and Electron Shielding YouTube Electron Shielding Down A Group electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Along the period from left to right along a. evaluate how electron shielding. Electron Shielding Down A Group.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Along the period from left to right along a. Complete electron shells shield the. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. Electron Shielding Down A Group.
From slideplayer.com
Section 3 Periodic Trends ppt download Electron Shielding Down A Group the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. electron shielding refers to the blocking of valence shell. Electron Shielding Down A Group.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e Electron Shielding Down A Group Along the period from left to right along a. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of electron shielding, in which. Electron Shielding Down A Group.
From slideplayer.com
Metals. Metals Scientists Families Vocab Trends. ppt download Electron Shielding Down A Group Complete electron shells shield the. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. the. Electron Shielding Down A Group.
From slidetodoc.com
The Periodic Table The how and why History Electron Shielding Down A Group shielding increases down a group because the nuclear core is farther removed from the valence electrons. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence. Electron Shielding Down A Group.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free Electron Shielding Down A Group evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an. Electron Shielding Down A Group.
From slideplayer.com
Periodic Trends OBJECTIVES ppt download Electron Shielding Down A Group electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Along the period from left to right along a. the concept of electron. Electron Shielding Down A Group.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. shielding increases down a group because the nuclear core is farther removed from the valence electrons. Complete electron shells shield the. evaluate how electron shielding contributes to periodic trends such as atomic. Electron Shielding Down A Group.
From slideplayer.com
Modern Atomic Theory (a.k.a. the electron chapter!) ppt download Electron Shielding Down A Group the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. Complete electron shells shield the. Along the period from left to right along a. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. evaluate how electron shielding contributes to periodic trends such. Electron Shielding Down A Group.
From www.slideserve.com
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free Electron Shielding Down A Group Along the period from left to right along a. shielding increases down a group because the nuclear core is farther removed from the valence electrons. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. electron affinity generally decreases down a group of elements because each atom is. Electron Shielding Down A Group.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID3652853 Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. shielding increases down a group because the nuclear core is farther removed from. Electron Shielding Down A Group.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Electron Shielding Down A Group Complete electron shells shield the. shielding increases down a group because the nuclear core is farther removed from the valence electrons. Along the period from left to right along a. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding refers to. Electron Shielding Down A Group.
From elchoroukhost.net
Alkali Metals Periodic Table Reactivity Elcho Table Electron Shielding Down A Group shielding increases down a group because the nuclear core is farther removed from the valence electrons. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the. Electron Shielding Down A Group.
From ar.inspiredpencil.com
Shielding Effect Trend In Periodic Table Electron Shielding Down A Group shielding increases down a group because the nuclear core is farther removed from the valence electrons. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. Electron Shielding Down A Group.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of. evaluate how electron shielding contributes to periodic trends such as atomic radius and. Electron Shielding Down A Group.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog Electron Shielding Down A Group shielding increases down a group because the nuclear core is farther removed from the valence electrons. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge. Electron Shielding Down A Group.
From slideplayer.com
Coulomb’s Law PERIODIC TRENDS Nuclear Charge Electron Shielding ppt Electron Shielding Down A Group Along the period from left to right along a. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Complete electron shells shield the. evaluate how electron shielding contributes to periodic trends such as atomic radius and ionization energy across a period and.. Electron Shielding Down A Group.
From slideplayer.com
Elemental Properties and Patterns ppt download Electron Shielding Down A Group the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. shielding increases down a group because the nuclear core. Electron Shielding Down A Group.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. shielding increases down a group because the nuclear core is farther removed from the valence electrons. . Electron Shielding Down A Group.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download Electron Shielding Down A Group the concept of the shielding effect explains the variation of atomic radius, ionization energy, etc. Along the period from left to right along a. electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. electron shielding refers to the blocking of valence. Electron Shielding Down A Group.
From slideplayer.com
New topic The Periodic Table ppt download Electron Shielding Down A Group electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend,. Along the period from left to right along a. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. . Electron Shielding Down A Group.