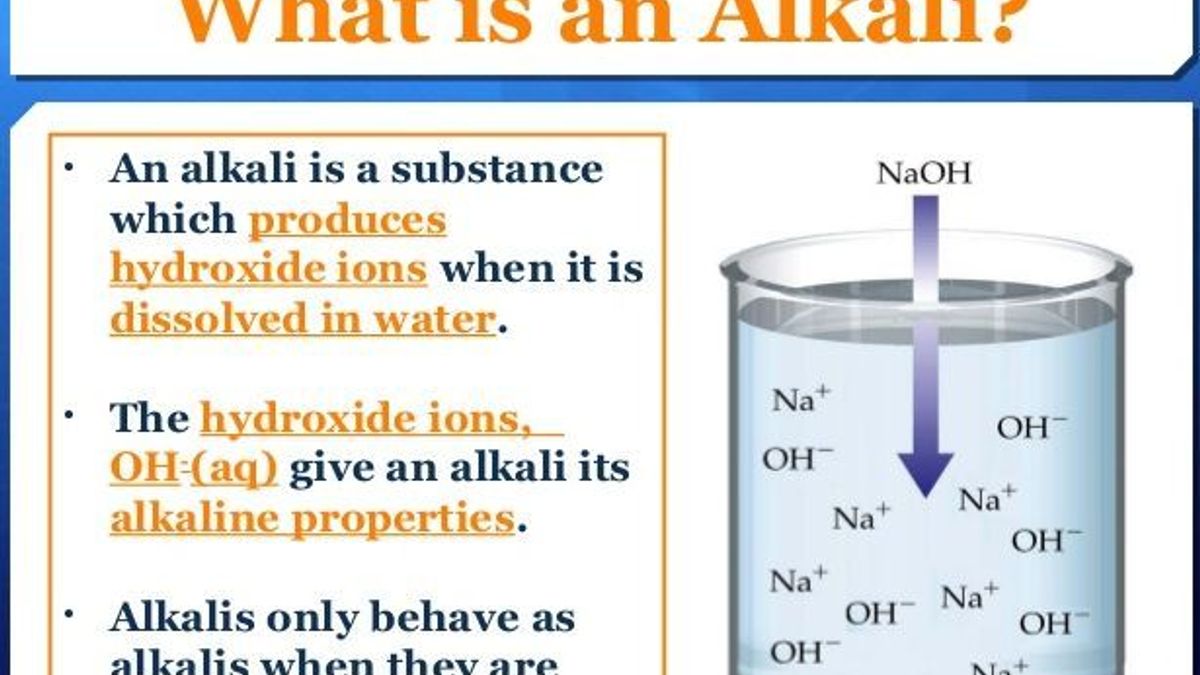
Do All Alkalis End In Hydroxide . A base that can dissolve in water is also called an alkali. Acids, bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. There is no special system for naming bases. When these substances react together in a neutralisation reaction, the h. A neutralisation reaction occurs when an acid reacts with an alkali; Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. Here are the ions involved in the. For sqa national 5 chemistry, learn about the.
from www.jagranjosh.com
Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. Acids and bases can neutralise each other. A base that can dissolve in water is also called an alkali. A neutralisation reaction occurs when an acid reacts with an alkali; Acids, bases and alkalis are found in the laboratory and at home. For sqa national 5 chemistry, learn about the. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. When these substances react together in a neutralisation reaction, the h. There is no special system for naming bases.
What is an Alkali and Alkaline solution?
Do All Alkalis End In Hydroxide When these substances react together in a neutralisation reaction, the h. There is no special system for naming bases. Acids, bases and alkalis are found in the laboratory and at home. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. When these substances react together in a neutralisation reaction, the h. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. Acids and bases can neutralise each other. A base that can dissolve in water is also called an alkali. A neutralisation reaction occurs when an acid reacts with an alkali; For sqa national 5 chemistry, learn about the. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. Here are the ions involved in the.
From www.slideserve.com
PPT Major stages in making chemicals PowerPoint Presentation, free download ID2499326 Do All Alkalis End In Hydroxide Acids, bases and alkalis are found in the laboratory and at home. Here are the ions involved in the. There is no special system for naming bases. A base that can dissolve in water is also called an alkali. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. Acids and bases can neutralise each other. Hydrochloric. Do All Alkalis End In Hydroxide.
From www.bbc.co.uk
Acids and Alkalis BBC Bitesize Do All Alkalis End In Hydroxide A neutralisation reaction occurs when an acid reacts with an alkali; When these substances react together in a neutralisation reaction, the h. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. For sqa national 5 chemistry, learn about the. Here are the ions involved in the. Acids and bases can neutralise each other. The hydroxide ions. Do All Alkalis End In Hydroxide.
From www.scribd.com
3.alkalis and Acids PDF Acid Hydroxide Do All Alkalis End In Hydroxide Here are the ions involved in the. Acids, bases and alkalis are found in the laboratory and at home. A neutralisation reaction occurs when an acid reacts with an alkali; When these substances react together in a neutralisation reaction, the h. There is no special system for naming bases. During the reaction, hydroxide ions will snatch a hydrogen back from. Do All Alkalis End In Hydroxide.
From pediaa.com
Difference Between Alkali and Metal Hydroxide Definition, Formation, Properties, Examples Do All Alkalis End In Hydroxide During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. For sqa national 5 chemistry, learn about the. There is no special system for naming bases. Acids, bases and alkalis are found in the laboratory and at home. When these substances react together in a neutralisation reaction, the h. The hydroxide ions of an alkali can react. Do All Alkalis End In Hydroxide.
From passnownow.com
SS1 Chemistry Third Term Bases and Alkalis Passnownow Do All Alkalis End In Hydroxide Here are the ions involved in the. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. Acids, bases and alkalis are found in the laboratory and at home. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium. Do All Alkalis End In Hydroxide.
From www.jagranjosh.com
What is an Alkali and Alkaline solution? Do All Alkalis End In Hydroxide Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. When these substances react together in a neutralisation reaction, the h. Acids and bases can neutralise each other. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. Here are the ions involved in the. During the reaction, hydroxide ions will. Do All Alkalis End In Hydroxide.
From slideplayer.com
OCR Gateway Additional Science ppt download Do All Alkalis End In Hydroxide Acids, bases and alkalis are found in the laboratory and at home. When these substances react together in a neutralisation reaction, the h. Here are the ions involved in the. A neutralisation reaction occurs when an acid reacts with an alkali; During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. For sqa national 5 chemistry, learn. Do All Alkalis End In Hydroxide.
From elchoroukhost.net
Alkali Metals Periodic Table Location Elcho Table Do All Alkalis End In Hydroxide When these substances react together in a neutralisation reaction, the h. A base that can dissolve in water is also called an alkali. There is no special system for naming bases. Acids and bases can neutralise each other. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. For sqa national 5 chemistry, learn about the. Acids,. Do All Alkalis End In Hydroxide.
From slideplayer.com
BIOCHEMISTRY. ppt download Do All Alkalis End In Hydroxide Here are the ions involved in the. When these substances react together in a neutralisation reaction, the h. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. A neutralisation reaction occurs when an acid reacts with an alkali; During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. Acids and bases can neutralise. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT Acids and Bases (3) PowerPoint Presentation, free download ID5525636 Do All Alkalis End In Hydroxide Acids and bases can neutralise each other. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. Acids, bases and alkalis are found in the laboratory and at home. Here are the ions involved in the. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. For sqa national 5 chemistry,. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT Acids and Bases (3) PowerPoint Presentation, free download ID4347436 Do All Alkalis End In Hydroxide For sqa national 5 chemistry, learn about the. A neutralisation reaction occurs when an acid reacts with an alkali; The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. There is no special system for naming bases. Acids and bases can neutralise each other. Here are the ions involved in the. Hydrochloric acid (\(\ce{hcl}\)),. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT Mrs Teocc PowerPoint Presentation, free download ID1776788 Do All Alkalis End In Hydroxide Here are the ions involved in the. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. When these substances react together in a neutralisation reaction, the h. For sqa national 5 chemistry, learn about the. Acids, bases and alkalis are found in the laboratory and at home. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)),. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT What are acids and alkalis? PowerPoint Presentation, free download ID5407263 Do All Alkalis End In Hydroxide There is no special system for naming bases. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. A base that can dissolve in water is also called an alkali. For sqa national 5 chemistry, learn about the. Acids, bases and alkalis are found in the laboratory and at home. Acids and bases can neutralise each other.. Do All Alkalis End In Hydroxide.
From www.shalom-education.com
Acids and Alkalis GCSE Chemistry Revision Do All Alkalis End In Hydroxide For sqa national 5 chemistry, learn about the. A neutralisation reaction occurs when an acid reacts with an alkali; A base that can dissolve in water is also called an alkali. Here are the ions involved in the. Acids, bases and alkalis are found in the laboratory and at home. There is no special system for naming bases. Hydrochloric acid. Do All Alkalis End In Hydroxide.
From spmchemistry.blog.onlinetuition.com.my
Physical Properties of Alkali Metals SPM Chemistry Do All Alkalis End In Hydroxide The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. Here are the ions involved in the. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. For sqa national 5 chemistry, learn about the. There is no special system for naming bases. When these substances react together in a neutralisation. Do All Alkalis End In Hydroxide.
From slideplayer.com
Periodicity Chapters 6 and ppt download Do All Alkalis End In Hydroxide Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. Here are the ions involved in the. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. There is no special system for naming bases. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. Acids, bases and. Do All Alkalis End In Hydroxide.
From www.youtube.com
Properties of Alkali and Alkaline Earth Metals Hydroxides, Chemistry Lecture Sabaq.pk YouTube Do All Alkalis End In Hydroxide Acids and bases can neutralise each other. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. There is no special system for naming bases. A base that can dissolve in water is also called an alkali. Acids, bases and alkalis are found in the laboratory and at home. Here are the ions involved in the. For. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT Acids and Alkalis PowerPoint Presentation, free download ID5037746 Do All Alkalis End In Hydroxide A neutralisation reaction occurs when an acid reacts with an alkali; Acids, bases and alkalis are found in the laboratory and at home. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. Acids and bases can neutralise each other. A base that can dissolve in water is also called an alkali. Here are the ions involved. Do All Alkalis End In Hydroxide.
From www.thoughtco.com
An Explanation of the Process Hydrolysis Do All Alkalis End In Hydroxide For sqa national 5 chemistry, learn about the. When these substances react together in a neutralisation reaction, the h. A neutralisation reaction occurs when an acid reacts with an alkali; The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. Acids. Do All Alkalis End In Hydroxide.
From www.slideshare.net
Acids and alkalis ppt1292425009 Do All Alkalis End In Hydroxide Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. When these substances react together in a neutralisation reaction, the h. Acids and bases can neutralise each other. A base that can dissolve in water is also called an alkali. Acids, bases and alkalis are found in the laboratory and at home. The hydroxide ions of an. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT What are acids and alkalis? PowerPoint Presentation, free download ID5407263 Do All Alkalis End In Hydroxide During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. A base that can dissolve in water is also called an alkali. When these substances react together in a neutralisation reaction, the h. For sqa national 5 chemistry, learn about the. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts.. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT Acids and Bases (3) PowerPoint Presentation, free download ID4347436 Do All Alkalis End In Hydroxide When these substances react together in a neutralisation reaction, the h. Acids, bases and alkalis are found in the laboratory and at home. There is no special system for naming bases. For sqa national 5 chemistry, learn about the. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. Acids and bases can neutralise each other. A. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT What are acids and alkalis? PowerPoint Presentation, free download ID6403983 Do All Alkalis End In Hydroxide Acids, bases and alkalis are found in the laboratory and at home. Here are the ions involved in the. A neutralisation reaction occurs when an acid reacts with an alkali; When these substances react together in a neutralisation reaction, the h. There is no special system for naming bases. A base that can dissolve in water is also called an. Do All Alkalis End In Hydroxide.
From www.scribd.com
Acids and Alkalis Notes PDF Hydroxide Ph Do All Alkalis End In Hydroxide For sqa national 5 chemistry, learn about the. Acids, bases and alkalis are found in the laboratory and at home. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. A base that can dissolve in water is also called an alkali. There is no special system for naming bases. Here are the ions involved in the.. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT Acids and Alkalis PowerPoint Presentation, free download ID5525043 Do All Alkalis End In Hydroxide Acids, bases and alkalis are found in the laboratory and at home. For sqa national 5 chemistry, learn about the. There is no special system for naming bases. When these substances react together in a neutralisation reaction, the h. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. The hydroxide ions of an alkali can react. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT Acids, Bases and Alkalis PowerPoint Presentation, free download ID3063336 Do All Alkalis End In Hydroxide The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. Acids, bases and alkalis are found in the laboratory and at home. A neutralisation reaction occurs when an acid reacts with an alkali; There is no special system for naming bases. When these substances react together in a neutralisation reaction, the h. A base. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT Acids and Bases (3) PowerPoint Presentation, free download ID5525636 Do All Alkalis End In Hydroxide Acids, bases and alkalis are found in the laboratory and at home. Here are the ions involved in the. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium. Do All Alkalis End In Hydroxide.
From studymind.co.uk
Group 1 Reactions (GCSE Chemistry) Study Mind Do All Alkalis End In Hydroxide Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. When these substances react together in a neutralisation reaction, the h. Acids, bases and alkalis are found in the laboratory and at home. Here are the ions involved in the. Acids and bases can neutralise each other. A base that can dissolve in water is also called. Do All Alkalis End In Hydroxide.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations* — the science sauce Do All Alkalis End In Hydroxide When these substances react together in a neutralisation reaction, the h. A base that can dissolve in water is also called an alkali. Acids and bases can neutralise each other. Here are the ions involved in the. A neutralisation reaction occurs when an acid reacts with an alkali; For sqa national 5 chemistry, learn about the. The hydroxide ions of. Do All Alkalis End In Hydroxide.
From www.pinterest.com
Alkali metals react with water to produce hydroxides. Alkali metal, Gcse chemistry, Chemistry Do All Alkalis End In Hydroxide A neutralisation reaction occurs when an acid reacts with an alkali; During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. Here are the ions involved in the. When these substances react together in a neutralisation reaction, the h. A base. Do All Alkalis End In Hydroxide.
From spmchemistry.blog.onlinetuition.com.my
Role of Water to Show Properties of Acids SPM Chemistry Do All Alkalis End In Hydroxide Acids, bases and alkalis are found in the laboratory and at home. A neutralisation reaction occurs when an acid reacts with an alkali; Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. Here are the ions involved in the. For sqa national 5 chemistry, learn about the. There is no special system for naming bases. During. Do All Alkalis End In Hydroxide.
From slideplayer.com
Learning Objectives Acids and Alkalis ppt download Do All Alkalis End In Hydroxide Here are the ions involved in the. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. A neutralisation reaction occurs when an acid reacts with an alkali; During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,.. Do All Alkalis End In Hydroxide.
From www.sliderbase.com
Acids and alkalis Presentation Chemistry Do All Alkalis End In Hydroxide For sqa national 5 chemistry, learn about the. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. Here are the ions involved in the. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. Acids and bases. Do All Alkalis End In Hydroxide.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID5525228 Do All Alkalis End In Hydroxide A base that can dissolve in water is also called an alkali. Hydrochloric acid (\(\ce{hcl}\)), nitric acid (\(\ce{hno3}\)), and carbonic acid (\(\ce{h2co3}\)) are all acids. Here are the ions involved in the. There is no special system for naming bases. The hydroxide ions of an alkali can react with the ammonium ions of these ammonium salts. During the reaction, hydroxide. Do All Alkalis End In Hydroxide.
From spmchemistry.blog.onlinetuition.com.my
Physical Properties of Alkali Metals SPM Chemistry Do All Alkalis End In Hydroxide For sqa national 5 chemistry, learn about the. Acids and bases can neutralise each other. There is no special system for naming bases. Here are the ions involved in the. Acids, bases and alkalis are found in the laboratory and at home. During the reaction, hydroxide ions will snatch a hydrogen back from ammonium ions,. A base that can dissolve. Do All Alkalis End In Hydroxide.