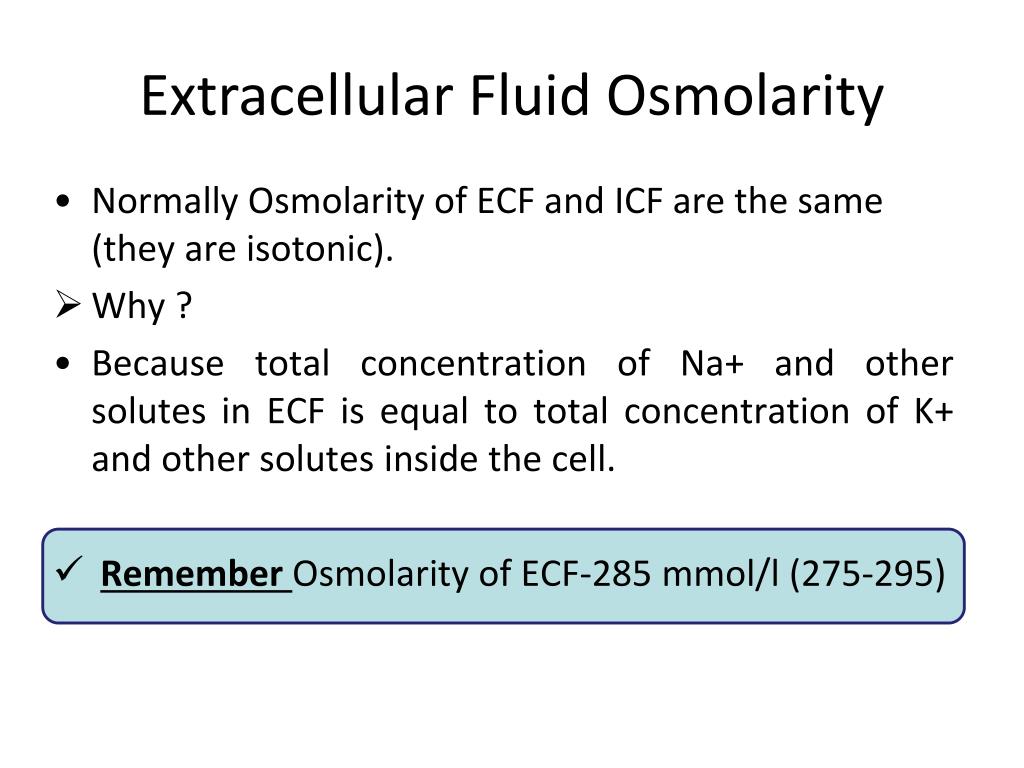
How To Calculate Ecf Osmolarity . To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Describes the movement of water through a semipermeable membrane. Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. The last part of the problem is to add the amount of the. Students use the new osmolarity to calculate the ecf and icf volumes. Calculated values are indicated by bold type. Why isn’t inorganic phosphate the primary ecf buffer? Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Calculate the serum tonicity or request a plasma osmolality. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Calculated tonicity and plasma osmolality are viewed as interchangeable.
from www.slideserve.com
Calculate the serum tonicity or request a plasma osmolality. Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Calculated tonicity and plasma osmolality are viewed as interchangeable. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Calculated values are indicated by bold type. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Students use the new osmolarity to calculate the ecf and icf volumes. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Why isn’t inorganic phosphate the primary ecf buffer? 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis.
PPT Body Fluids PowerPoint Presentation, free download ID2152192
How To Calculate Ecf Osmolarity Why isn’t inorganic phosphate the primary ecf buffer? Describes the movement of water through a semipermeable membrane. Calculated values are indicated by bold type. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Calculated tonicity and plasma osmolality are viewed as interchangeable. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. The last part of the problem is to add the amount of the. Calculate the serum tonicity or request a plasma osmolality. Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Why isn’t inorganic phosphate the primary ecf buffer? Students use the new osmolarity to calculate the ecf and icf volumes.
From www.slideserve.com
PPT Body Fluids PowerPoint Presentation, free download ID2152192 How To Calculate Ecf Osmolarity Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Calculated tonicity and plasma osmolality are viewed as interchangeable. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Why isn’t inorganic phosphate the primary ecf buffer? Calculated values are indicated by bold type. Students use the new osmolarity to calculate the ecf. How To Calculate Ecf Osmolarity.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube How To Calculate Ecf Osmolarity The last part of the problem is to add the amount of the. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Why isn’t inorganic phosphate the primary ecf buffer? Calculated values are indicated by. How To Calculate Ecf Osmolarity.
From www.youtube.com
How to solve osmolarity calculation problems 4 YouTube How To Calculate Ecf Osmolarity To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Students use the new osmolarity to calculate the ecf and icf volumes.. How To Calculate Ecf Osmolarity.
From www.youtube.com
How to solve osmolarity calculation problems 2 YouTube How To Calculate Ecf Osmolarity Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Students use the new osmolarity to calculate the ecf and icf volumes. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Why isn’t inorganic phosphate the primary ecf buffer? Describes the movement of water through a semipermeable. How To Calculate Ecf Osmolarity.
From www.slideserve.com
PPT Regulation of Extracellular Fluid Osmolarity and Sodium Concentration PowerPoint How To Calculate Ecf Osmolarity Students use the new osmolarity to calculate the ecf and icf volumes. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Why isn’t inorganic phosphate the primary ecf buffer? Calculate the serum tonicity or request a plasma osmolality. Calculate the approximate ecf volume of a patient after administration of 3.0. How To Calculate Ecf Osmolarity.
From ibiologia.com
Osmolarity Definition, Formula & Osmolarity vs. Osmolality How To Calculate Ecf Osmolarity Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Calculated tonicity and plasma osmolality are viewed as interchangeable. Calculated values are indicated by bold type. Calculate the serum tonicity or request a plasma osmolality. Students use the new osmolarity to calculate the ecf and icf volumes. Why isn’t inorganic phosphate the primary. How To Calculate Ecf Osmolarity.
From www.studypool.com
SOLUTION 6 regulation of extracellular fluid osmolarity and sodium concentration Studypool How To Calculate Ecf Osmolarity Calculated values are indicated by bold type. Describes the movement of water through a semipermeable membrane. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Calculate the serum tonicity or request a plasma osmolality. Students use the new osmolarity to calculate the ecf and icf volumes. To equalize osmolarity, water moves between the ecf and the icf,. How To Calculate Ecf Osmolarity.
From fyoplrsjt.blob.core.windows.net
How Do You Estimate Osmolarity at Pauline Holman blog How To Calculate Ecf Osmolarity Calculated tonicity and plasma osmolality are viewed as interchangeable. Describes the movement of water through a semipermeable membrane. Why isn’t inorganic phosphate the primary ecf buffer? The last part of the problem is to add the amount of the. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. 20% of body weight,. How To Calculate Ecf Osmolarity.
From www.slideserve.com
PPT Body Fluids PowerPoint Presentation, free download ID2152192 How To Calculate Ecf Osmolarity Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Why isn’t inorganic phosphate the primary ecf buffer? The last part of the problem is to add the amount of the. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Students use. How To Calculate Ecf Osmolarity.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download ID4759369 How To Calculate Ecf Osmolarity Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Calculated tonicity and plasma osmolality are viewed as interchangeable. Students use the new osmolarity to calculate the ecf and icf volumes. Why isn’t inorganic phosphate the primary ecf buffer? Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Calculate the. How To Calculate Ecf Osmolarity.
From www.slideshare.net
Body fluids new How To Calculate Ecf Osmolarity 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Calculated tonicity and plasma osmolality are viewed as interchangeable. The last. How To Calculate Ecf Osmolarity.
From studydemandants.z21.web.core.windows.net
How To Calculate Plasma Osmolarity How To Calculate Ecf Osmolarity Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Calculate the serum tonicity or request a plasma osmolality. Calculated values are indicated by bold type. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Describes the movement. How To Calculate Ecf Osmolarity.
From www.slideserve.com
PPT Body Fluids PowerPoint Presentation, free download ID2152192 How To Calculate Ecf Osmolarity Describes the movement of water through a semipermeable membrane. Calculated tonicity and plasma osmolality are viewed as interchangeable. The last part of the problem is to add the amount of the. Students use the new osmolarity to calculate the ecf and icf volumes. Why isn’t inorganic phosphate the primary ecf buffer? Lower concentration than bicarbonate (~ 1.5 mmol / l). How To Calculate Ecf Osmolarity.
From www.thetechedvocate.org
How to calculate osmolarity The Tech Edvocate How To Calculate Ecf Osmolarity Calculated values are indicated by bold type. Describes the movement of water through a semipermeable membrane. Students use the new osmolarity to calculate the ecf and icf volumes. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Describe. How To Calculate Ecf Osmolarity.
From www.slideserve.com
PPT Body Fluid Compartments & Physiological Solutions PowerPoint Presentation ID3290236 How To Calculate Ecf Osmolarity To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. The last part of the problem is to add the amount of the. Why isn’t inorganic phosphate the primary ecf buffer? Calculate the serum tonicity or request a plasma osmolality. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not. How To Calculate Ecf Osmolarity.
From www.youtube.com
Fluid balance 2 ECF osmolarity YouTube How To Calculate Ecf Osmolarity Why isn’t inorganic phosphate the primary ecf buffer? Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Students use the new osmolarity to calculate the ecf and icf volumes. 20%. How To Calculate Ecf Osmolarity.
From www.youtube.com
How to solve osmolarity calculation problems 3 YouTube How To Calculate Ecf Osmolarity Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. To equalize osmolarity, water moves between. How To Calculate Ecf Osmolarity.
From www.studypool.com
SOLUTION 6 regulation of extracellular fluid osmolarity and sodium concentration Studypool How To Calculate Ecf Osmolarity Calculated values are indicated by bold type. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. The last part of the problem is to add the amount of the. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. 20% of body weight, or 1/3 of total body. How To Calculate Ecf Osmolarity.
From calculatorshub.net
Fluid Osmolarity Calculator Online How To Calculate Ecf Osmolarity Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Describes the movement of water through a semipermeable membrane. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Calculated tonicity and plasma osmolality are viewed as interchangeable. Calculated values are. How To Calculate Ecf Osmolarity.
From www.slideserve.com
PPT Regulation of Extracellular Fluid Osmolarity and Sodium Concentration PowerPoint How To Calculate Ecf Osmolarity Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Calculated tonicity and plasma osmolality are viewed as interchangeable. Calculate the serum tonicity or request a plasma osmolality. Describes the movement of water through a semipermeable membrane. Students use the new osmolarity to calculate the ecf and icf. How To Calculate Ecf Osmolarity.
From www.youtube.com
Using VolumeOsmolality Diagrams to Understand Body Fluid Status YouTube How To Calculate Ecf Osmolarity Describes the movement of water through a semipermeable membrane. Calculate the serum tonicity or request a plasma osmolality. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Calculate the approximate ecf volume of a patient. How To Calculate Ecf Osmolarity.
From www.youtube.com
Regulation of Extracellular Fluid Osmolarity Physiology Online Lectures VLearning YouTube How To Calculate Ecf Osmolarity Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. 20% of body weight, or 1/3 of. How To Calculate Ecf Osmolarity.
From www.youtube.com
Renal system 13 Osmolarity How to calculate osmotic pressure Van't Hoff law YouTube How To Calculate Ecf Osmolarity 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Calculated values are indicated by bold type. Why isn’t inorganic phosphate the primary ecf buffer? Calculated tonicity and plasma osmolality are viewed as interchangeable. Calculate the serum tonicity or request a plasma osmolality. The last part of the problem is to add the amount of. How To Calculate Ecf Osmolarity.
From www.chegg.com
Solved Q3 The intracellular osmolarity is 300 mOsM. Which How To Calculate Ecf Osmolarity Describes the movement of water through a semipermeable membrane. Why isn’t inorganic phosphate the primary ecf buffer? The last part of the problem is to add the amount of the. Calculated tonicity and plasma osmolality are viewed as interchangeable. Students use the new osmolarity to calculate the ecf and icf volumes. Calculated values are indicated by bold type. Calculate the. How To Calculate Ecf Osmolarity.
From www.youtube.com
Calculating the Osmolarity of a Solution YouTube How To Calculate Ecf Osmolarity Students use the new osmolarity to calculate the ecf and icf volumes. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Why isn’t inorganic phosphate the primary ecf buffer? The last part of the problem is to add the amount of the. To equalize osmolarity, water moves between the ecf and the icf, from. How To Calculate Ecf Osmolarity.
From www.youtube.com
Calculating the Osmolarity of a Solution (Question 2) YouTube How To Calculate Ecf Osmolarity Calculated values are indicated by bold type. Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Describes the movement of water through a semipermeable membrane. Calculate the serum tonicity or request a plasma osmolality. Why. How To Calculate Ecf Osmolarity.
From www.slideshare.net
Introduction to body fluid & cell membrane How To Calculate Ecf Osmolarity The last part of the problem is to add the amount of the. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Calculated values are indicated by bold type. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. To equalize osmolarity, water moves between the ecf and the icf, from areas. How To Calculate Ecf Osmolarity.
From www.drawittoknowit.com
Physiology Glossary ECF Volume Contraction & Expansion Draw It to Know It How To Calculate Ecf Osmolarity Students use the new osmolarity to calculate the ecf and icf volumes. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water. How To Calculate Ecf Osmolarity.
From hxebamyle.blob.core.windows.net
How To Calculate Osmolarity From Percent Solution at Carol Reyes blog How To Calculate Ecf Osmolarity Calculate the serum tonicity or request a plasma osmolality. Why isn’t inorganic phosphate the primary ecf buffer? The last part of the problem is to add the amount of the. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Students. How To Calculate Ecf Osmolarity.
From www.chegg.com
Solved A 100 kg male with a normal ECF osmolarity (300 mOsm) How To Calculate Ecf Osmolarity Describes the movement of water through a semipermeable membrane. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Calculated values are indicated by bold type. The last part of the. How To Calculate Ecf Osmolarity.
From www.youtube.com
Osmolarity Example (Bio) YouTube How To Calculate Ecf Osmolarity The last part of the problem is to add the amount of the. Calculate the serum tonicity or request a plasma osmolality. Why isn’t inorganic phosphate the primary ecf buffer? Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas. How To Calculate Ecf Osmolarity.
From www.slideserve.com
PPT Intracellular vs. extracellular concentrations PowerPoint Presentation ID945667 How To Calculate Ecf Osmolarity 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Students use the new osmolarity to calculate the ecf and icf volumes. Describes the movement of water through a semipermeable membrane. To equalize osmolarity, water moves between the ecf and the icf, from areas of lower osmolarity to areas of. Calculate the approximate ecf volume. How To Calculate Ecf Osmolarity.
From www.youtube.com
BODY FLUID VOLUME OSMOLARITY DIAGRAM YouTube How To Calculate Ecf Osmolarity Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. Calculate the serum tonicity or request a plasma osmolality. Describes the movement of water through a semipermeable membrane. The last part of the problem is to add the amount of the. Why isn’t inorganic phosphate the primary ecf. How To Calculate Ecf Osmolarity.
From www.youtube.com
How to Calculate the Plasma Osmolarity YouTube How To Calculate Ecf Osmolarity Calculate the serum tonicity or request a plasma osmolality. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose solution, assuming complete metabolism of the. The last part of the problem is to add the amount of. How To Calculate Ecf Osmolarity.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry YouTube How To Calculate Ecf Osmolarity Lower concentration than bicarbonate (~ 1.5 mmol / l) is not volatile;. Describe how the tonicity (osmolality) of the extracellular fluid is maintained byalterations in water intake and vasopressin. 20% of body weight, or 1/3 of total body water, is extracellular fluid (ecf) osmosis. Calculate the approximate ecf volume of a patient after administration of 3.0 l of 5% glucose. How To Calculate Ecf Osmolarity.