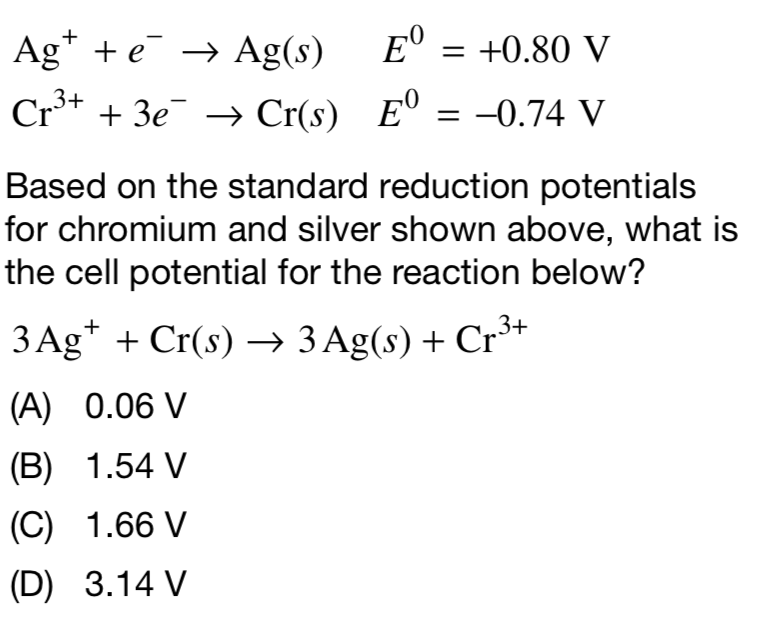Standard Cell Potential Of Ag . The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Determine the standard deviation of e0 using. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Since the definition of cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode.
from www.chegg.com
Determine the standard deviation of e0 using. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode.
Solved Ag+ + e→ Ag(s) Cr3+ + 3e→ Cr(s) +0.80 V E,0 =0.74
Standard Cell Potential Of Ag The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Determine the standard deviation of e0 using. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Since the definition of cell.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Standard Cell Potential Of Ag Determine the standard deviation of e0 using. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure. Standard Cell Potential Of Ag.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Standard Cell Potential Of Ag Since the definition of cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Determine the standard deviation of e0 using.. Standard Cell Potential Of Ag.
From www.chegg.com
Solved 5. What is the standard cell potential (E cel) for Standard Cell Potential Of Ag Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Plot e(m) as a function. Standard Cell Potential Of Ag.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID5581588 Standard Cell Potential Of Ag Since the definition of cell. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Determine the standard deviation of. Standard Cell Potential Of Ag.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential Of Ag Since the definition of cell. Determine the standard deviation of e0 using. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an. Standard Cell Potential Of Ag.
From www.chegg.com
Solved Ag+ + e→ Ag(s) Cr3+ + 3e→ Cr(s) +0.80 V E,0 =0.74 Standard Cell Potential Of Ag Since the definition of cell. Determine the standard deviation of e0 using. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an. Standard Cell Potential Of Ag.
From www.slideserve.com
PPT Biopotential Electrodes PowerPoint Presentation, free download ID9337323 Standard Cell Potential Of Ag Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Since the definition of cell. Determine the standard deviation of e0 using. The potential of the cell under standard conditions (1 m for solutions,. Standard Cell Potential Of Ag.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID7041672 Standard Cell Potential Of Ag Since the definition of cell. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Plot e(m) as a function. Standard Cell Potential Of Ag.
From www.chegg.com
Solved Part A Calculate the standard potential for the Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. Since the definition of cell. Determine the standard deviation of e0 using. The potential of the cell under standard conditions (1 m for solutions,. Standard Cell Potential Of Ag.
From byjus.com
2 The standard half reduction potential of Ag+Ag is 0.79V is 25^° C. Given the experimental Standard Cell Potential Of Ag The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Since the definition of cell. Determine the standard deviation of e0 using. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode.. Standard Cell Potential Of Ag.
From www.chegg.com
Solved Part A Calculate the standard potential for the Standard Cell Potential Of Ag Determine the standard deviation of e0 using. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode.. Standard Cell Potential Of Ag.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Magnesium/Silver Galvanic Cell Nagwa Standard Cell Potential Of Ag Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between. Standard Cell Potential Of Ag.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Of Ag Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Since the definition of cell. Determine the standard deviation of e0 using. The potential of the cell under standard conditions (1 m for solutions,. Standard Cell Potential Of Ag.
From www.pveducation.org
Standard Potential PVEducation Standard Cell Potential Of Ag Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Plot e(m) as a function. Standard Cell Potential Of Ag.
From www.chegg.com
Solved The standard potential of the cell Pt (s) H2 (g) Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and. Standard Cell Potential Of Ag.
From www.numerade.com
SOLVED (i) Use the information in the Resource section to calculate the standard potential of Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Determine the standard deviation of e0 using. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure. Standard Cell Potential Of Ag.
From www.echemi.com
Where does the free electron from from in Ag/AgCl reaction? ECHEMI Standard Cell Potential Of Ag Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure. Standard Cell Potential Of Ag.
From www.bartleby.com
Answered 31)Calculate the cell potential for the… bartleby Standard Cell Potential Of Ag Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Determine the standard deviation of e0 using.. Standard Cell Potential Of Ag.
From www.numerade.com
SOLVED Two reduction potential reactions and their standard reduction potentials are given Standard Cell Potential Of Ag Since the definition of cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Determine the standard deviation of e0 using.. Standard Cell Potential Of Ag.
From www.numerade.com
152. Draw a diagram like Figure 156 to convert the following potentials. The Ag AgCl and Standard Cell Potential Of Ag Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and. Standard Cell Potential Of Ag.
From 2012books.lardbucket.org
Standard Potentials Standard Cell Potential Of Ag The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. Since the definition of cell. The cell potential, \(e_{cell}\), is the measure. Standard Cell Potential Of Ag.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Electrode Potentials Nagwa Standard Cell Potential Of Ag Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and. Standard Cell Potential Of Ag.
From www.numerade.com
SOLVED 1 Calculate the standard cell potential (from the Standard Reduction Potentials Table Standard Cell Potential Of Ag Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Determine the standard deviation of e0 using. The cell potential, \(e_{cell}\), is. Standard Cell Potential Of Ag.
From www.numerade.com
SOLVED Cell Potentials The standard potential of a voltaic cell can be calculated from the Standard Cell Potential Of Ag Since the definition of cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure. Standard Cell Potential Of Ag.
From www.chegg.com
Solved Part A Calculate the standard potential for the Standard Cell Potential Of Ag Determine the standard deviation of e0 using. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. Since the definition of cell. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions,. Standard Cell Potential Of Ag.
From byjus.com
32. Ksp of AGI is 16. Standard reduction electrode potential of Ag is 0.8V. Find the the EMF for Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Plot e(m) as a function of m1⁄2 and determine the. Standard Cell Potential Of Ag.
From question.pandai.org
Standard Electrode Potential Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and. Standard Cell Potential Of Ag.
From chem.libretexts.org
6.5 Standard Reduction Potentials Chemistry LibreTexts Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Determine the standard deviation of e0 using. Plot e(m) as. Standard Cell Potential Of Ag.
From www.chegg.com
Solved Calculate the standard cell potential for the Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Determine the standard deviation of. Standard Cell Potential Of Ag.
From www.numerade.com
SOLVEDkalculate the standard cell potential of the following cell at 25C Zn( 8) Zn? + (aq) Cu Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Since the definition of cell. Determine the standard deviation of e0 using. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c). Standard Cell Potential Of Ag.
From www.semanticscholar.org
[PDF] Evaluation of Ag/AgClelectrode standard potential uncertainty used in primary pH Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or. Standard Cell Potential Of Ag.
From users.highland.edu
Standard Potentials Standard Cell Potential Of Ag The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Determine the standard deviation of e0 using. Since the definition of cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode.. Standard Cell Potential Of Ag.
From www.researchgate.net
Electrode potential curves vs. Ag/AgCl reference electrodes in the... Download Scientific Diagram Standard Cell Potential Of Ag The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and. Standard Cell Potential Of Ag.
From www.researchgate.net
Standard reduction potential (V vs Ag/AgCl) of different elements in... Download Scientific Standard Cell Potential Of Ag Plot e(m) as a function of m1⁄2 and determine the standard potential of the ag agcl electrode. Determine the standard deviation of e0 using. Since the definition of cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called.. Standard Cell Potential Of Ag.
From www.chegg.com
Solved The cell potential for the standard galvanic cell Standard Cell Potential Of Ag Since the definition of cell. Determine the standard deviation of e0 using. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an. Standard Cell Potential Of Ag.